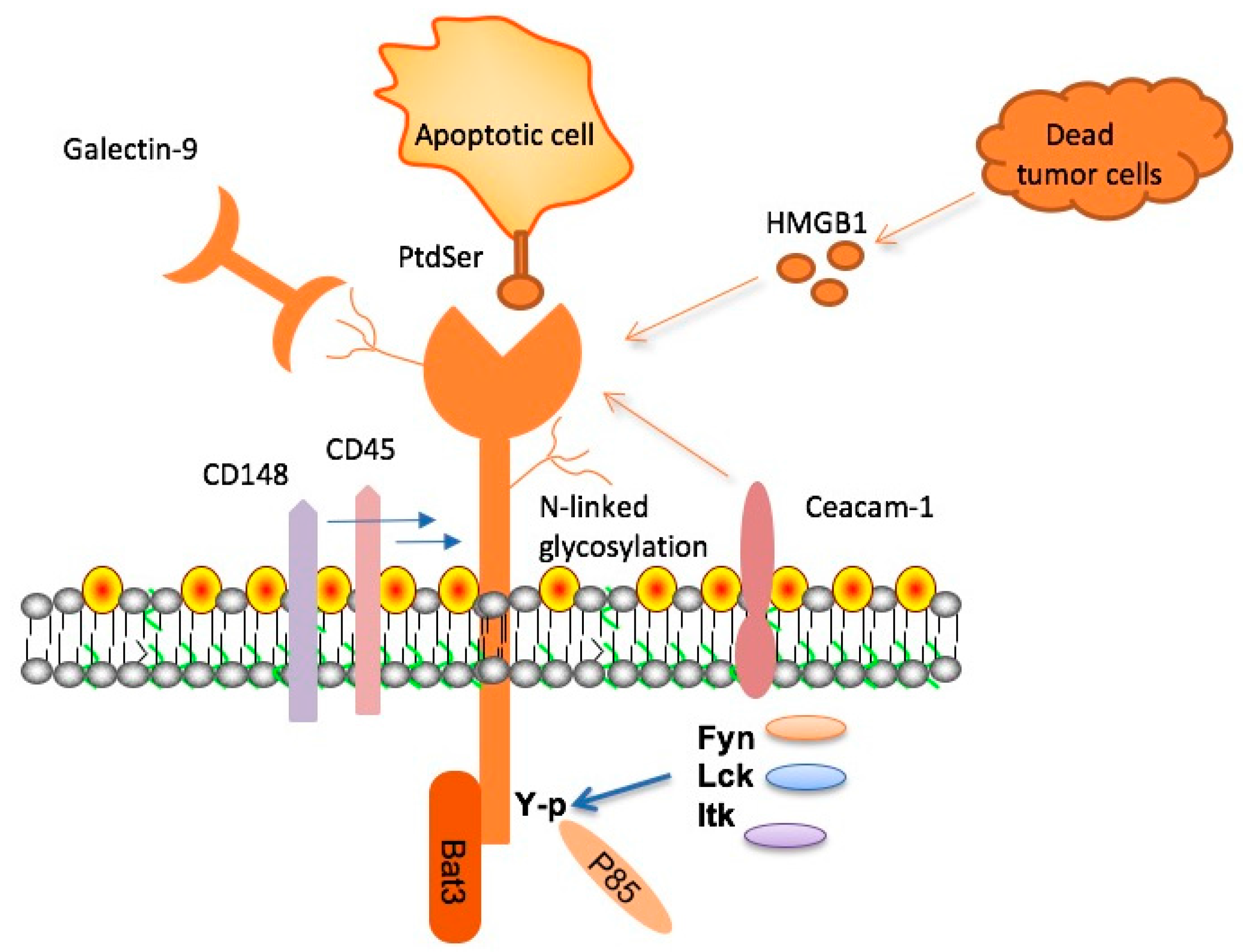
TIM-3 therapy for Alzheimer’s is an innovative approach that leverages the immune system’s mechanisms to combat Alzheimer’s disease, a condition that afflicts millions worldwide. Recent research highlights the potential of TIM-3, a checkpoint molecule, to enable microglia – the brain’s immune cells – to effectively clear toxic amyloid plaques linked to cognitive decline. This breakthrough is particularly significant as it aligns with developments in Alzheimer’s disease research that suggest harnessing immune strategies can enhance cognitive function improvement. By releasing the inhibitory effects of TIM-3, scientists aim to rejuvenate microglia, restoring their ability to facilitate plaque clearance and memory enhancement. As we explore the intricate connection between the immune system and Alzheimer’s, TIM-3 therapy may emerge as a promising candidate in the landscape of treatment strategies.
The exploration of TIM-3 in Alzheimer’s treatment reflects broader innovations in addressing neurodegenerative diseases through immune modulation. By targeting checkpoint molecules similar to those used in cancer therapies, researchers are investigating how therapeutic strategies aimed at this genetic factor can lead to advancements in cognitive health. Additionally, the unique role of microglia in maintaining brain homeostasis while combating plaque formation by amyloid beta underscores the delicate balance necessary for cognitive preservation. This situation invites further inquiry into how immune dysfunction contributes to Alzheimer’s, potentially reinforcing the relationship between immunological health and neurodegeneration. With studies like these paving the way, they challenge conventional approaches and stimulate a new paradigm in Alzheimer’s disease management.
The Role of TIM-3 in Alzheimer’s Disease Therapy
Recent research highlights the potential of TIM-3 therapy for Alzheimer’s disease, revealing that blocking this immune checkpoint molecule could enhance the ability of microglia to target and remove amyloid plaques from the brain. When researchers deleted the TIM-3 gene in a laboratory setting, they observed that mice demonstrated improved cognitive function and plaque clearance. This finding is significant because it suggests that TIM-3 plays a restrictive role in the brain’s immune response, and by inhibiting it, we may be able to shift the immune system’s balance toward more effectively combating Alzheimer’s pathology.
The therapeutic strategy surrounding TIM-3 focuses on its dual function within the immune system; while it is crucial for regulating T cell activity to prevent autoimmunity, its overexpression in Alzheimer’s patients hinders microglial functionality. By utilizing anti-TIM-3 antibodies or other small molecules that block its action, researchers could potentially restore microglial activity, facilitating the clearance of harmful plaques and leading to significant cognitive improvements. This approach may usher in new, targeted Alzheimer’s treatments that effectively harness the body’s immune mechanisms.
Understanding Microglia’s Role in Alzheimer’s Disease
Microglia are the brain’s primary immune cells, and they perform essential roles in maintaining healthy cognitive function. In Alzheimer’s disease, however, the role of microglia becomes complicated. Elevated levels of TIM-3 inhibit their function, preventing the clearing of amyloid beta plaques that contribute to cognitive decline. Understanding this relationship highlights the need for innovative strategies, such as TIM-3 therapy, to restore normal microglial activity without compromising their critical functions in the brain.
In the context of Alzheimer’s disease research, it is crucial to explore the dual role of microglia not only in plaque removal but also in synaptic pruning and maintaining neurological health. Typically, microglia help eliminate excess synapses, fine-tuning circuits critical for memory and learning. With aging and the onset of Alzheimer’s, however, their function becomes impaired due to molecular signals like TIM-3, underscoring the significance of targeting these pathways for therapeutic interventions.
The Connection Between the Immune System and Alzheimer’s Disease
Emerging findings emphasize the intricate connection between the immune system and Alzheimer’s disease progression. As checkpoint molecules such as TIM-3 regulate immune responses, they can also impact the brain’s susceptibility to plaque accumulation. By understanding how the immune system interacts with Alzheimer’s pathology, researchers can identify new therapeutic avenues to modulate these interactions and potentially enhance cognitive function in patients.
Research into the immune system’s role in Alzheimer’s disease further underscores the potential for developing innovative treatments that can simultaneously address neuroinflammation and cognitive decline. By leveraging knowledge from cancer immunotherapy, where checkpoint inhibitors have shown promise, similar strategies may enhance the body’s immune response in combating Alzheimer’s, as studies suggest TIM-3 blockade could lead to improved microglial activity and plaque clearance.
Cognitive Function Improvement through TIM-3 Inhibition
The inhibition of TIM-3 has been associated with significant improvements in cognitive function in experimental models of Alzheimer’s disease. By allowing microglia to resume their natural role in clearing amyloid plaques, cognitive behaviors in mice were restored, resembling conditions prior to plaque accumulation. This breakthrough suggests that TIM-3 may be a critical target for therapeutic intervention aimed at not only halting progression but also reversing some cognitive deficits associated with Alzheimer’s.
Strategies focused on TIM-3 inhibition pave the way for a new class of Alzheimer’s treatments. These could potentially yield substantial benefits, especially considering the limited success of existing therapies. By prompting microglial activation, TIM-3 targeted treatments may restore cognitive abilities, offering hope where other strategies have fallen short. As pharmaceutical advancements progress, this mechanism holds promise for enhancing the quality of life for Alzheimer’s patients.
Current Strategies in Alzheimer’s Disease Research
Amid numerous drug trial failures in Alzheimer’s treatment, novel strategies leveraging immune pathways are gaining traction. Research focusing on checkpoint molecules like TIM-3 represents a paradigm shift in the understanding of Alzheimer’s pathology, providing fresh insights into manipulating the immune response to facilitate plaque clearance. Trials assessing the effectiveness of TIM-3 inhibitors are anticipated to yield critical data that could lead to viable solutions for challenging Alzheimer’s cases.
The emphasis on immune modulation through TIM-3 therapy underscores a broader trend in Alzheimer’s disease research that seeks to identify and exploit immune checkpoints for potential cognitive intervention. As new findings emerge, the prospect of integrating immunotherapy alongside conventional approaches may redefine treatment methodologies, creating a more comprehensive arsenal to combat Alzheimer’s and improve patient outcomes.
Future Implications of TIM-3 Research in Alzheimer’s Therapy
The implications of TIM-3 research are far-reaching and may transform Alzheimer’s therapeutics. As scientists explore the potential of TIM-3 inhibition, they are starting to bridge knowledge from immunology and neuroscience, potentially leading to groundbreaking treatments for Alzheimer’s that harness the body’s own immune responses. This could not only mitigate symptoms but also address the underlying mechanisms contributing to the disease, promising a future where Alzheimer’s can be effectively managed.
Looking ahead, the size and scope of ongoing studies into TIM-3 signaling pathways are crucial for determining the precise effects on human populations. Current investigations involving mouse models will help refine these treatment modalities, bringing researchers closer to human clinical applications. By understanding how TIM-3 inhibition can enhance microglial functionality, future therapies might significantly alter the landscape of Alzheimer’s disease management.
Mechanisms of Alzheimer’s Disease Progression
The mechanisms driving Alzheimer’s disease progression are complex and multifaceted, involving a range of biological processes. Central to this is the accumulation of amyloid plaques, which are not effectively cleared by microglia due to inhibitory signals like TIM-3. This accumulation leads to neuroinflammation and neuronal damage, underscoring the importance of understanding how to manipulate these pathways for effective treatment.
Research into the molecular intricacies of Alzheimer’s disease reveals the role of genetic factors in influencing disease severity and progression. Studies have shown that a heightened expression of TIM-3 in individuals with the disease correlates with cognitive decline, suggesting that targeting this molecule could restore lost cognitive functions. Comprehensive exploration of these mechanisms is vital for deciphering potential therapeutic interventions.
Clinical Trials and the Future of Alzheimer’s Treatments
The journey toward effective Alzheimer’s treatments is increasingly focused on clinical trials investigating TIM-3 inhibitors. With early results demonstrating promising outcomes in pre-clinical models, there is optimism about advancing these therapies to human trials. The unique role of TIM-3 in modulating immune responses presents a compelling case for its potential in restoring cognitive function and improving quality of life for patients.
As clinical trials continue to unfold, researchers are assessing not only efficacy but also long-term effects of TIM-3 targeted therapies. Understanding the pharmacodynamics and safety profile of these inhibitors will be paramount as they move toward approval processes. Future research directions will undoubtedly focus on optimizing these therapies to maximize benefits for Alzheimer’s patients and provide new hope in the fight against this debilitating disease.
Challenges and Considerations in Alzheimer’s Research
Despite the exciting prospects of TIM-3 as a therapeutic target in Alzheimer’s disease, challenges remain. Understanding the specific mechanisms by which TIM-3 affects microglial activity is crucial, as overly tampering with immune responses could lead to unforeseen consequences, including potential neuroinflammation. Balancing efficacy with safety is paramount in this evolving research landscape.
Furthermore, the variability of Alzheimer’s presentation among patients complicates the development of universally applicable treatments. As researchers strive to tailor TIM-3 therapies to individual genetic profiles, personalized approaches may emerge, maximizing effectiveness and minimizing risks. Continued exploration of the immune system’s role in Alzheimer’s will be critical for overcoming these hurdles and advancing therapeutic strategies.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s and how does it work?
TIM-3 therapy for Alzheimer’s targets the TIM-3 molecule, which inhibits microglia, the brain’s immune cells, from clearing amyloid plaques. By blocking TIM-3, this therapy frees microglia to attack and remove these plaques, which can improve cognitive function and memory.
How does TIM-3 relate to Alzheimer’s disease research?
TIM-3 is linked to late-onset Alzheimer’s disease through genetic studies. Research has shown that high TIM-3 expression on microglia leads to reduced plaque clearance, making it a significant target in Alzheimer’s disease research for restoring cognitive function.
Could TIM-3 therapy improve cognitive function in Alzheimer’s patients?
Yes, TIM-3 therapy has been demonstrated to enhance cognitive function in animal models of Alzheimer’s. By allowing microglia to effectively clear amyloid plaques, TIM-3 inhibition may lead to memory improvement in patients.
What role do microglia play in TIM-3 therapy for Alzheimer’s?
Microglia are the immune cells of the brain responsible for clearing away harmful plaques. In TIM-3 therapy, inhibiting TIM-3 enables these crucial cells to attack amyloid plaques more effectively, which is essential for treating Alzheimer’s disease.
What are the implications of TIM-3 therapy for future cancer treatment strategies?
While TIM-3 therapy primarily focuses on Alzheimer’s, its approach of blocking immune checkpoint molecules may offer insights into developing more effective cancer treatment strategies by enhancing immune responses against tumors, given TIM-3’s role in regulating immune activity.
How long did the research into TIM-3 therapy for Alzheimer’s take?
The research on TIM-3 therapy for Alzheimer’s spanned five years, focusing on the genetic implications and the impact of TIM-3 on microglial function, leading to potential therapeutic applications in human conditions.
What evidence supports the effectiveness of TIM-3 therapy in Alzheimer’s?
Evidence from mouse models indicates that deleting the TIM-3 gene enhances plaque clearance and improves cognitive behaviors. This suggests that TIM-3 therapy could be a viable option to improve cognitive function in Alzheimer’s patients.
What is the next step in TIM-3 therapy research for Alzheimer’s patients?
The next step is testing human anti-TIM-3 antibodies in mouse models of Alzheimer’s to evaluate their effectiveness in preventing plaque formation, paving the way for clinical applications in treating Alzheimer’s disease.
Is TIM-3 only relevant for Alzheimer’s disease or can it be applied to other conditions?
While TIM-3 is particularly relevant in Alzheimer’s disease due to its role in immune regulation and plaque clearance, its mechanisms may also provide insights for other neurodegenerative conditions and autoimmune diseases where immune checkpoints are involved.
What challenges exist in delivering TIM-3 therapy to human patients with Alzheimer’s?
Challenges include ensuring that TIM-3 inhibitors effectively reach the brain, as some therapies targeting amyloid plaques have previously failed due to vascular complications. Research is focusing on developing targeted therapies that minimize risks while maximizing therapeutic effects.
| Key Points | Details |
|---|---|
| TIM-3 Therapy for Alzheimer’s | The therapy utilizes TIM-3 molecule deletion to enhance clearance of plaques and restore memory. |
| Study Findings | Mice without TIM-3 had improved memory as microglia could clear Alzheimer’s plaques. |
| Role of TIM-3 | TIM-3 inhibits microglia from attacking harmful amyloid plaques in the brain. |
| Research Duration | The study took five years, involving multiple experiments and collaborators. |
| Potential Human Application | Proposed treatments include anti-TIM-3 antibodies or small molecules to block TIM-3’s function. |
Summary
The research into TIM-3 therapy for Alzheimer’s disease offers promising insights into potential new treatment avenues. By deleting the TIM-3 molecule, scientists can free microglial cells in the brain to clear away harmful plaques, resulting in improved cognitive functions in affected models. This groundbreaking approach not only highlights the interconnectedness of immune checkpoints in both cancer and Alzheimer’s, but it also reinforces the potential of TIM-3 as a viable target for therapeutic intervention, making TIM-3 therapy a noteworthy consideration in the battle against Alzheimer’s.