
Memory Formation Techniques: A New Insight into Synaptic Plasticity
Memory formation techniques are essential to understanding how our brains encode and retain information. Recent advancements in neuroscience research, particularly the exploration of synaptic plasticity, have shed light on the intricate processes that underpin learning and memory. Researchers have identified new methods for mapping these processes, which could offer hope for preventing or treating neurodegenerative disorders that affect memory, such as Alzheimer’s disease. Among these innovative strategies is the EPSILON technique, which allows scientists to observe the movements of key proteins in neurons that play a pivotal role in synaptic functioning. By gaining insights into the molecular foundations of memory, we have the potential to develop therapies that significantly improve cognitive health and resilience in aging populations.
When diving into the realm of cognitive memory techniques, it’s fascinating to explore how our brain constructs and retrieves memories. Synaptic behavior and plasticity are fundamental concepts that illuminate the mechanisms behind learning capacity and memory retention. Innovative research methods are increasingly shining a light on these complex interactions, paving new paths for tackling issues related to cognitive decline. Techniques such as the Extracellular Protein Surface Labeling in Neurons (EPSILON) play a critical role in investigating the molecular basis of memory, especially concerning the vital proteins that facilitate neuron communication. This exploration not only highlights the importance of understanding our brain’s functionality but also underscores the significance of developing effective strategies to combat memory-related disorders.
Understanding Memory Formation Techniques
Memory formation techniques are crucial in neuroscience, especially as researchers delve into the complex mechanisms of learning and retention. One such innovative method is known as the EPSILON technique. Developed by a Harvard research team, EPSILON allows scientists to map the proteins responsible for synaptic transmission, specifically in relation to learning and memory processes. By illuminating the synaptic architecture, this technique uncovers how information is encoded within the brain’s neural networks, shedding light on the dynamic nature of memory formation.
The importance of understanding these techniques cannot be overstated. Neuroscience research highlights synaptic plasticity, which refers to the brain’s ability to strengthen or weaken synapses based on activity levels — a fundamental process in learning. By utilizing advanced microscopy and fluorescent labeling, researchers can observe these changes in real time, offering insights into how new memories are formed, retrieved, and stored. As scientists refine these techniques, they pave the way for developing innovative treatments for neurodegenerative disorders like Alzheimer’s, where memory impairment is prevalent.
The Role of Synaptic Plasticity in Learning and Memory
At the heart of learning and memory is the concept of synaptic plasticity, a process that allows synapses, the connections between neurons, to adjust their strength based on experience. Recent research has demonstrated the significance of this dynamic ability, particularly through studies involving the EPSILON technique. By mapping the behavior of AMPARs, key proteins in synaptic plasticity, researchers are revealing the intricate patterns that dictate which connections are enhanced or diminished during memory formation.
Synaptic plasticity not only influences how memories are formed but also plays a critical role in cognitive functions across various contexts. Understanding this process deepens our comprehension of learning, from mundane tasks to complex problem-solving. With findings from neuroscience research indicating that disrupted synaptic function can lead to neurodegenerative disorders, ongoing investigations into synaptic plasticity hold promise for the advancement of therapeutic strategies aimed at restoring cognitive health.
Innovations in Neurological Research: The EPSILON Technique
The EPSILON technique represents a groundbreaking advancement in neurological research, providing unparalleled insights into synaptic behavior at the molecular level. By focusing on the movements of AMPARs, researchers can observe how these proteins interact and change during memory-related processes. This high-resolution approach allows scientists to visualize the real-time dynamics of synaptic interactions, permitting a deeper understanding of how memories are encoded within the brain’s complex neural circuitry.
Such innovations extend beyond mere curiosity; they hold the potential to transform how we understand and treat neurological conditions. As research continues to apply the EPSILON technique across various cognitive studies, the implications for developing new therapies targeting disorders like dementia become clearer. The ability to map synaptic plasticity and memory formation intricacies may lead to the identification of novel intervention strategies, underscoring the critical role that foundational research plays in health advancements.
Linking Protein Dynamics to Memory Trajectories
Investigating the relationship between protein dynamics and memory trajectories is pivotal in unraveling the mysteries of how memories are formed and retained. The application of the EPSILON technique enables researchers to link the trafficking of AMPARs to the expression of immediate early genes like cFos, which serve as indicators of gene activation in response to particular stimuli. This correlation provides valuable insights into the biological underpinnings of memory formation and the activation of specific neural circuits.
Further exploration into these protein dynamics not only enhances our understanding of memory processes but also highlights potential markers for memory retention and retrieval. By examining these dynamics in the context of neurodegenerative disorders, researchers can identify critical points of intervention, potentially leading to therapeutic advancements. This intricate interplay between protein movement and memory dynamics underscores the complexity of cognitive functions and the ongoing need for innovative research techniques.
Future Directions for Memory Research
As researchers expand upon the foundational insights provided by the EPSILON technique, the future of memory research looks promising. This new technique is offering scientists a more detailed understanding of how synaptic changes correlate with different types of memories. By mapping synaptic potentiation and history over time, researchers can better outline the dynamics of memory formation, which may unlock new strategies for combating memory-related disorders.
Improving therapeutic approaches to memory impairments involves not only understanding normal cognitive processes but also identifying how these processes go awry in neurodegenerative conditions. As EPSILON is adopted worldwide, researchers will likely discover new avenues for enhancing synaptic health and restoring cognitive functionality, providing hope for individuals affected by diseases like Alzheimer’s. The support of continued research efforts in this arena is essential for progress.
The Impact of Neuroscience Research on Cognitive Health
Neuroscience research plays a vital role in enhancing our understanding of cognitive health, particularly as it relates to the complexities of memory formation and learning. Breakthrough techniques such as EPSILON provide crucial insights into the molecular mechanisms governing synaptic plasticity. These discoveries not only inform our understanding of how memories are constructed but also illuminate the pathological processes involved in conditions like dementia.
The knowledge gained from these investigations is instrumental in developing targeted interventions that can address cognitive decline. By focusing on the core processes of synaptic reinforcement and plasticity, future research can help mitigate the impact of neurodegenerative disorders. Ultimately, the intersection of research and practical application will be paramount in forging the path toward improved therapies and patient outcomes in brain health.
Synaptic Mechanisms and Memory Encoding
Understanding the synaptic mechanisms involved in memory encoding is critical for appreciating how the brain processes and retains information. The EPSILON technique has unveiled new ways to observe these mechanisms, allowing researchers to closely monitor the interactions between neurotransmitter receptors like AMPARs and the formation of memories. These findings illustrate how specific synaptic changes contribute to the retention of both short and long-term memories.
Furthermore, exploring these synaptic mechanisms provides insights into various types of learning scenarios, revealing how distinct memories are structured within the brain. This nuanced understanding of memory encoding not only enhances our comprehension of normal cognitive functioning but also helps identify irregularities associated with neurodegenerative diseases. By layering these insights with practical applications, researchers continue to lay the groundwork for innovative treatment options.
Lessons from Synaptic Dysfunction in Neurodegenerative Disorders
The investigation of synaptic dysfunction in neurodegenerative disorders offers valuable lessons in the broader context of memory comprehension. Research reveals that conditions like Alzheimer’s involve significant alterations in synaptic plasticity, leading to impaired memory formation and retention. Utilizing techniques such as EPSILON helps clarify how these synaptic changes can be tracked and understood, contributing to our knowledge about the disease mechanisms at play.
By studying the specifics of synaptic impairment, researchers can better identify potential therapeutic targets. Understanding the pathways involved in synaptic modification is critical for developing interventions that may slow or halt cognitive decline. Continued research into these issues holds the potential for groundbreaking therapies that can improve quality of life for individuals affected by neurodegenerative disorders.
The Role of Advanced Microscopy in Neuroscience Discoveries
Advanced microscopy techniques are revolutionizing the way scientists explore neurological phenomena, especially in studying memory formation and synaptic interactions. With the development of the EPSILON method, researchers can examine synapses at unprecedented resolutions, unveiling details about synaptic behavior that were previously unattainable. This level of precision allows for significant insights into the biological foundations of learning and retention.
The application of such advanced tools extends far beyond mere observation; it enables researchers to form hypotheses about synaptic function and impairment in real time. As neuroscientific discoveries build upon these innovations, they provide a clearer understanding of cognitive health and the underlying mechanisms at play in both learning and neurodegenerative conditions. Such advancements underscore the critical importance of continuous investment in cutting-edge research technologies.
Frequently Asked Questions
What are Memory Formation Techniques and how do they relate to synaptic plasticity?
Memory Formation Techniques refer to the strategies and methods employed to enhance the process of encoding and retaining information in the brain. These techniques are closely linked to synaptic plasticity, which is the brain’s ability to strengthen or weaken synapses based on experience. Research has shown that understanding and utilizing these techniques can significantly improve learning and memory through the modulation of synaptic connections.
How does the EPSILON technique improve our understanding of memory formation?
The EPSILON technique, or Extracellular Protein Surface Labeling in Neurons, is a groundbreaking method that allows for the detailed mapping of synapses involved in memory formation. By illuminating the proteins, specifically AMPARs, that are crucial for synaptic plasticity, EPSILON provides unprecedented insights into how memories are created and retained, helping researchers understand the underlying mechanisms of learning and memory.
What role does synaptic plasticity play in learning and memory?
Synaptic plasticity is fundamental to learning and memory as it involves the strengthening or weakening of synaptic connections based on experiences. This dynamic adjustment allows the brain to adapt and reorganize itself, making it possible to store new information effectively. Memory Formation Techniques aim to harness this process to enhance cognitive performance and therapeutic outcomes for individuals with memory impairments.
Can Memory Formation Techniques help in understanding neurodegenerative disorders?
Yes, Memory Formation Techniques can provide critical insights into neurodegenerative disorders such as Alzheimer’s disease. By studying synaptic plasticity through techniques like EPSILON, researchers can identify the synaptic dysfunctions that contribute to memory loss in these conditions. This understanding can lead to the development of new therapeutic approaches aimed at enhancing memory and cognitive function.
What findings did the EPSILON technique reveal regarding AMPARs in memory formation?
The EPSILON technique revealed that AMPARs, which are vital for synaptic plasticity, are closely linked to memory traces or engrams in the brain. The research indicated that the trafficking of these receptors is crucial for the formation and retention of enduring memories, highlighting the interplay between protein dynamics and cognitive processes.
How does researching memory formation contribute to therapy for dementia patients?
Researching memory formation through advanced techniques like EPSILON helps us understand the molecular and synaptic mechanisms involved in learning and memory. By deciphering these processes, scientists can develop targeted therapies that aim to restore synaptic integrity and enhance memory function in dementia patients, potentially improving their quality of life.
What future applications can arise from the EPSILON technique in neuroscience research?
The EPSILON technique holds promise for various future applications in neuroscience research. It can be used to explore different types of memories and their specific patterns of synaptic plasticity. This method allows researchers to investigate cognitive phenomena more deeply, and its molecular tools may also facilitate the development of innovative strategies to treat memory impairments and cognitive decline.
| Key Points | Details |
|---|---|
| Groundbreaking Techniques | Harvard researchers have developed a new method called EPSILON to map how learning and memories are formed. |
| Molecular Mapping | The EPSILON technique illuminates the synaptic architecture of memory at unprecedented resolution. |
| Importance of Synapses | Synapses are the junctions where neurons communicate and are crucial for memory formation. |
| Key Proteins | AMPARs are essential proteins that play a vital role in synaptic plasticity. |
| Research Findings | Initial applications of EPSILON have revealed new insights into memory traces in the brain. |
| Future Applications | EPSILON may aid in understanding cognitive phenomena and improving therapies for memory impairments. |
Summary
Memory Formation Techniques are pivotal in understanding how we learn and recall information. The recent advancements in techniques like EPSILON showcase how we can map synaptic interactions at an unprecedented level of detail. This not only highlights the underlying processes of memory formation but also paves the way for innovative treatments for neurological disorders such as dementia. By continuing to explore these techniques, researchers can deepen our understanding of the brain and enhance strategies to combat memory-related ailments.

Gene Editing: Exploring the Promise and Ethical Challenges
Gene editing has emerged as a groundbreaking advancement in modern science, spearheaded by innovative tools like CRISPR technology. This revolutionary capability allows for precise modifications of genetic material, opening doors to potential cures for debilitating diseases, such as using a sickle cell cure that could transform lives. However, the rapid progression of gene editing raises significant ethical questions that society must confront, particularly regarding the long-term implications for health equity and the medical ethics surrounding such interventions. As we consider the promises offered by gene editing, it is equally critical to address the associated risks that come with altering the human genome. The debate on the ethics of gene editing encapsulates our responsibility in determining the future of genetic alteration amid the potential for enhanced health outcomes.
The field of genetic modification, best exemplified by CRISPR, is revolutionizing how we approach medical conditions that previously seemed insurmountable. By enabling targeted edits to DNA, scientists can potentially eradicate genetic disorders, underscoring the promise of a new era in healthcare. Yet, with such remarkable power comes a host of complex considerations, especially the ethical dilemmas that challenge our viewpoints on what it means to be human. As advancements in genetic tools bring us closer to the prospect of curing conditions like sickle cell disease, we must also engage in difficult conversations about health justice and the implications of these technologies on societal well-being. This discourse compels us to reflect on the balance between innovation and the moral responsibilities that accompany the ability to alter our genetic destiny.
Understanding Gene Editing: The Promise of CRISPR Technology
Gene editing has revolutionized our approach to treating genetic disorders, particularly through the use of CRISPR technology. With the capability to precisely alter DNA sequences, CRISPR offers a beacon of hope for conditions that were once deemed untreatable. The process involves utilizing the Cas9 enzyme alongside a guide RNA to identify and modify specific genes, providing the potential to cure diseases like sickle cell anemia. The advancements in gene editing create an exciting landscape for medical science, but as we tread further into this new territory, it raises critical questions about the implications of altering human genetics.
The immediate promise of CRISPR technology is evident in the development of potential cures for serious conditions. For example, researchers are now able to effectively remove the genetic mutation that leads to sickle cell anemia, a painful disease that affects approximately 100,000 individuals in the U.S. However, alongside this prospect lies the ethical dilemma of how far should gene editing go. As Baer pointed out, while curing life-threatening diseases is one thing, contemplating edits for conditions that do not pose an immediate health threat – or that define our individuality – is much more complex.
Ethical Dilemmas: Navigating the Complexities of Gene Editing
The ethics of gene editing are as significant as the technology itself, requiring careful consideration of both the medical and social ramifications. Who is entitled to make decisions about gene alterations? Should parents have the authority to ‘design’ their children’s traits, such as intelligence or physical abilities? These questions emerge critically in discussions surrounding the use of CRISPR, pushing us to engage in a deeper exploration of medical ethics. The debate is intensified when contemplating interventions for conditions like Down syndrome, raising pressing inquiries about value judgments in the healthcare community.
Additionally, the socio-economic implications of gene editing cannot be overlooked. As advancements in CRISPR technology become increasingly commercialized, issues of health equity arise. The staggering cost of treatments, such as the estimated $2.2 million for sickle cell cures, poses a dilemma about access to these innovations. Wealthier populations may benefit disproportionately from these breakthroughs, leading to broader societal inequities. As Rebecca Weintraub Brendel notes, innovation must not only be scientifically sound but must also consider the ethical implications on health justice, ensuring that technology does not exacerbate existing gaps in healthcare access.
The Future of Healthcare: Gene Editing’s Role in Curing Diseases
As we navigate the future of healthcare, gene editing stands out as a transformative tool in the fight against genetic diseases. The potential to edit somatic cells to treat conditions like sickle cell disease is groundbreaking. Innovations from researchers such as Sekar Kathiresan illustrate the capabilities of gene manipulation to lower LDL cholesterol effectively. However, these advances also need critical assessment, as we must understand the broader implications such edits may have on the body. The complexity of gene interactions and the potential consequences of insights from the past 3 billion years of evolutionary biology highlight that while some changes seem beneficial on the surface, they could introduce unforeseen complications.
The promise of curing diseases through gene editing must also be balanced with a responsibility for overseeing the technology. As news of gene editing has spread, so too have concerns about unregulated practices in other countries. The importance of establishing robust regulatory frameworks that not only protect human subjects but also maintain ethical standards in medical practice is paramount. The journey towards effectively utilizing gene editing technologies must be taken with caution, ensuring developments in the medical field prioritize safety and ethical considerations alongside scientific innovation.
Health Equity in Gene Editing: A Critical Analysis
Health equity is an essential consideration in the realm of gene editing. As technologies like CRISPR provide opportunities to cure diseases, there exists a real danger of creating a healthcare divide where only affluent populations receive the benefits of scientific advancements. The disparity in access to groundbreaking treatments raises critical questions about justice in healthcare. For conditions like sickle cell anemia, which predominantly affects marginalized communities, ensuring that all individuals have access to these therapies is not just a health issue; it’s a societal obligation.
Moreover, the ethical aspect of health equity must involve not just access to treatments, but the larger narrative of who dictates which diseases are prioritized for research and funding. The tension between providing care for those who can afford it and ensuring that innovative treatments are accessible to all populations is pivotal. Thus, ongoing discussions about health equity should not overlook the voices of those affected by genetic disorders, ensuring that solutions are inclusive and comprehensively address disparities.
Cultural Perspectives on Gene Editing: A Global Debate
Gene editing does not exist in a vacuum; it is entwined with cultural and societal perspectives that vary worldwide. Discussions around the ethics of gene editing could change dramatically based on cultural values, particularly regarding what constitutes a ‘desirable’ genetic trait. In some cultures, when we consider interventions that alter ‘human variation’ — such as those involving deafness or other non-life-threatening conditions — the emphasis may be less on ‘fixing’ a condition and more on recognizing and celebrating diversity. This perspective is crucial as we move forward in defining what gene editing should aim to achieve.
Furthermore, international dialogues around gene editing can provide different insights into regulatory approaches. Countries with contrasting stances on gene editing ethics may influence global standards or practices. For instance, while some regions may impose strict regulations against germline editing, others might advocate for its exploration. Thus, fostering a global discourse on gene editing ethics must accommodate varied cultural attitudes, ensuring that advancements in science uphold shared human dignity and respect for diverse beliefs.
The Role of Medical Ethics in Gene Editing
Medical ethics plays a crucial role in the debate surrounding gene editing, particularly as the technology rapidly evolves. Practitioners and researchers must grapple with the implications of their work, ensuring that patient welfare remains paramount. Ethical guidelines must be established to help navigate the complexities of gene manipulation, balancing the pursuit of scientific knowledge with the responsibility to safeguard human rights and dignity. As seen in discussions surrounding CRISPR, the dialogue extended beyond the lab and into societal realms, underscoring the need for ethical frameworks that govern practice and research in this field.
In addition, the role of the medical community extends to advocating for patient voices in discussions about gene editing. Patients’ experiences with genetic disorders should inform ethical decision-making and offer valuable insights into the impacts of gene editing on real lives. This accountability to those directly affected will help in crafting ethical frameworks that prioritize human well-being and ensure that innovative therapies contribute positively to the health care system.
The Intersection of Gene Editing and Medical Research
The intersection of gene editing and medical research is an exciting prospect that can lead to groundbreaking therapies. Innovations driven by CRISPR technology have the potential to accelerate the pace of research, opening doors to new understanding of genetic conditions and their treatment. By enabling precise modifications to DNA, researchers can study gene function in ways previously thought unattainable. This exploratory nature of gene editing also paves the way for personalized medicine, where treatments can be tailored to individual genetic profiles, enhancing efficacy and safety.
However, the integration of gene editing into medical research must be matched with rigorous scientific inquiry to unravel potential risks and maintain ethical standards. Each breakthrough carries responsibilities, with researchers obligated to conduct thorough risk assessments before proceeding with any clinical applications. The dual focus on innovation and caution will define the trajectory of gene editing research, as we ensure that the promise of these technologies aligns with the principles of medical ethics and patient safety.
Cost and Accessibility: Navigating the Financial Implications of Gene Editing
The financial implications of gene editing are a major concern as we consider its adoption in clinical settings. With the prices of cutting-edge treatments soaring — such as the $2.2 million cost for sickle cell cures — the question arises of who can afford such life-saving interventions. The potential for gene editing to widen existing health disparities is real, posing challenges for policymakers and healthcare providers aiming to ensure equitable access. Access to advanced therapies could hinge on socioeconomic status, creating a healthcare landscape divided by affordability.
Addressing the intersection of cost and accessibility involves proactive measures to develop policies that promote fairness in managing gene editing technologies. Innovative funding models, public health initiatives, and advocacy are essential components to bridge the affordability gap. Ensuring that every patient, regardless of financial means, has access to transformative gene therapies is imperative for achieving true health equity, transforming the promise of gene editing into a reality for all.
Public Perception of Gene Editing: Navigating Societal Concerns
Public perception is a critical component of the gene editing conversation, shaping how society views and interacts with emerging technologies. As CRISPR and similar tools generate media attention, differential opinions reflect varying degrees of trust in scientific advancements. While some celebrate gene editing as a revolutionary leap forward in medicine, others express concerns about potential misuse and ethical violations. Addressing societal apprehensions is essential for legitimizing the dialogue surrounding the use of gene editing in health care.
Educational initiatives play a significant role in shaping public understanding of gene editing. As researchers and ethical committees work to elucidate the complexities of CRISPR and its applications, engagement with lay audiences becomes paramount. Clear communication of the benefits, risks, and ethical considerations associated with gene editing can foster informed discussions and help to demystify the technology. Building trust through transparency and education will be crucial as we navigate the future of gene editing in society.
Frequently Asked Questions
What is CRISPR technology and how does it relate to gene editing?
CRISPR technology is a revolutionary gene editing tool that allows scientists to modify DNA by adding, removing, or altering genetic material at specified locations in the genome. This technology, which stands for Clustered Regularly Interspaced Short Palindromic Repeats, enables precise gene editing, making it a cornerstone of modern genetics and offering potential cures for genetic diseases, such as sickle cell anemia.
What are the ethical concerns surrounding gene editing with CRISPR?
The ethics of gene editing involve complex issues, including the implications of altering human genetics, the potential for unintended consequences, and the societal impacts of such technologies. Questions arise about who should make decisions regarding gene editing, particularly for conditions that are not life-threatening, like Down syndrome, and the fairness of access to these potentially expensive therapies.
Could CRISPR technology be the key to a cure for sickle cell disease?
Yes, CRISPR technology offers promising potential as a cure for sickle cell disease by enabling scientists to edit the faulty genes responsible for the disorder. Recent advancements have demonstrated that manipulating somatic cells can successfully remove the genes underlying sickle cell disease in affected individuals, providing hope for effective treatments and possibly even cures.
How does health equity factor into the discussion of gene editing?
Health equity is a significant concern in the context of gene editing, especially with expensive treatments like those offered for sickle cell disease. The high costs of gene therapy raise questions about accessibility and fairness—will only those who can afford these treatments benefit, while others are left behind? Ensuring that advancements in gene editing benefit everyone, regardless of socioeconomic status, is critical for ethical progress in this field.
What are the potential unintended consequences of gene editing?
Gene editing, while transformative, carries risks associated with unintended consequences. Modifications to genes can disrupt natural genomic functions, potentially leading to unforeseen health issues. For example, changes made to genes that have evolved over billions of years can interact in complex ways with other biological processes, complicating the expected benefits and posing risks to long-term health.
| Key Point | Details |
|---|---|
| Gene Editing Overview | CRISPR technology allows precise changes to DNA, both in somatic and germline cells, raising possibilities for curing genetic diseases. |
| Ethical Dilemmas | Questions arise about the ethics of gene editing for non-lethal conditions like Down syndrome, and who determines what changes should be made. |
| Cost and Accessibility | Treatments like the sickle cell cure can cost $2.2 million, raising issues of fairness and health equity among different populations. |
| Parental Decisions | The right of parents to choose genetic attributes for their children is debated, especially in cases of non-pathological traits. |
| Regulatory Concerns | There are risks of unethical applications and lack of oversight in countries with looser regulations on gene editing. |
| Unintended Consequences | Gene interactions are complex, and changes can lead to unexpected health issues that are difficult to predict. |
Summary
Gene editing represents a revolutionary approach in medicine, providing the potential to cure genetic disorders and improve lives. At the same time, it poses significant ethical questions regarding the implications of altering human genetics. The discussion surrounding CRISPR technology, as highlighted by experts, emphasizes the importance of thoughtful consideration before embracing its applications. As we tread further into this frontier, it is crucial to address the associated costs, accessibility, and the moral responsibilities we carry towards future generations.

Health Tracking: Understanding Learning and Memory Formation
Health Tracking is increasingly essential in understanding the complex mechanisms behind learning and memory formation. Recent advancements in neuroscience research have shed light on the intricate processes of synaptic plasticity, the foundation for how we acquire and retain information. By utilizing innovative techniques such as the EPSILON method, researchers are uncovering vital insights that could pave the way for effective dementia treatment. This technique allows for a meticulous mapping of synaptic connections, highlighting the dynamics that govern memory storage. As we delve deeper into these scientific breakthroughs, Health Tracking not only enhances our comprehension of cognitive functions but also lays the groundwork for potential new therapies.
The domain of health monitoring encompasses a variety of approaches designed to enhance our understanding of cognitive processes. Through the lens of innovative methodologies, scientists are exploring how our brains develop learning and memories, particularly in light of challenges presented by neurodegenerative conditions. Concepts tied to synaptic restructuring and the manipulation of neuronal pathways are gaining traction, further emphasizing the need for effective strategies in combating memory-related disorders. Techniques such as EPSILON are not merely academic innovations; they represent potential breakthroughs in the realm of healthcare, particularly for conditions like dementia. By investigating these neurological frameworks, researchers aim to revolutionize treatment options and improve the lives of those affected by cognitive decline.
Understanding Learning and Memory Formation
The intricate processes involved in learning and memory creation hinge upon a complex network of neurons communicating via synapses. Within this network, synaptic plasticity plays a pivotal role, allowing for the adaptation and reorganization of neural connections in response to new experiences. Researchers are increasingly focusing on this phenomenon, as it directly influences the ways we retain information and how memories are recalled later. To understand memory formation deeply, it is essential to study not only the synapses themselves but also the molecular underpinnings that govern their dynamics.
At the forefront of neuroscience research, innovative techniques like the EPSILON method have emerged. This novel approach enables scientists to map the movement of essential proteins, such as AMPARs, which are critical in facilitating synaptic communication. By utilizing advanced microscopy alongside fluorescent labeling, researchers can now observe synaptic changes with unprecedented clarity, illuminating the relationship between synaptic plasticity and memory formation. Such insights may offer invaluable knowledge not just on learning processes, but also on the development of effective therapies for conditions like dementia, where these mechanisms deteriorate.
The Role of Synaptic Plasticity in Memory
The concept of synaptic plasticity is fundamental to understanding how memories are formed and retained. This adaptive capability of synapses enables the brain to strengthen certain neural pathways while weakening others, based on experiences. Research has demonstrated that changes in synaptic strength can significantly influence how memories are encoded and retrieved. The ability to study these processes in detail can vastly improve our understanding of cognitive functions and how they may be disrupted in various neurological disorders.
Recent advancements in methodologies, particularly with the introduction of the EPSILON technique, have further clarified the role of AMPARs in synaptic plasticity. By examining how these proteins are trafficked and located within the synapses, researchers are uncovering the rules that dictate synaptic modifications during memory formation. Such findings not only bolster our grasp of healthy brain function but also have implications for developing therapeutic strategies tailored to combat memory-related disorders, such as those seen in Alzheimer’s disease and other forms of dementia.
Health Tracking: Innovations in Neuroscience
Recent innovations in health tracking are paving the way for enhanced monitoring of cognitive health and memory function. With the advent of sophisticated techniques like EPSILON, health professionals and researchers can now track changes in synaptic plasticity over time, gaining insights into both normal cognitive function and memory impairments. This capability will enable more personalized medicine approaches, where treatments can be tailored based on an individual’s specific neurological profile.
Additionally, the integration of health tracking technologies with neuroscience research may help in the early detection and management of cognitive decline. By continuously monitoring synaptic changes, we could potentially identify the onset of conditions like dementia before significant symptoms appear. Such proactive strategies could revolutionize how we approach neurological health, shifting from reactive treatment to preventive care, significantly improving outcomes for patients suffering from cognitive disorders.
The EPSILON Technique and Its Impact on Research
The EPSILON technique represents a significant breakthrough in neuroscience research, enabling detailed observation of protein dynamics within neurons. By focusing on how AMPARs interact at synaptic junctions, researchers are able to elucidate the molecular mechanisms that underlie memory storage and retrieval. This precision not only reveals the intricacies of synaptic behavior but also opens doors for exploration into how these mechanisms can be altered during disease states.
Furthermore, the application of EPSILON extends beyond basic research into potential clinical settings. Understanding how synaptic plasticity is regulated can inform new treatment strategies for memory disorders, suggesting pathways for therapeutic intervention. As the scientific community continues to adopt and refine this technique, the prospects for innovating dementia treatment and other cognitive health strategies appear increasingly promising.
Neuroscience Research: Bridging Basic Science and Application
Neuroscience research often occupies a unique position between theoretical understanding and practical application. Advancements such as the EPSILON technique showcase how foundational research can lead to tangible benefits in health care. For instance, investigating the molecular pathways involved in learning and memory paves the way for developing new therapeutic strategies for conditions characterized by memory dysfunction.
Researchers like Adam Cohen stress the importance of supporting basic science as it lays the groundwork for future medical innovations. The sequential progress from understanding synaptic plasticity to applying that knowledge in clinical settings illustrates the interconnectedness of research fields. By fostering a robust research environment, the potential to advance treatments for cognitive disorders continues to grow, ultimately enhancing patient care and outcomes.
Emerging Therapies for Memory Impairments
The ongoing evolution of therapeutic strategies aimed at addressing memory impairments represents a critical area in neuroscience research. As understandings of conditions like Alzheimer’s deepen, so too do the approaches to treatment. Techniques like EPSILON provide not only insights into normal cognitive processes but also highlight potential therapeutic targets within synaptic pathways—offering hope for effective dementia treatment options.
In the near future, therapeutic developments may leverage findings from synaptic research to create interventions that enhance synaptic plasticity or restore lost functions. These advancements could significantly alter the landscape of memory impairment treatments, leading to improved quality of life for individuals afflicted with these challenging cognitive disorders. As researchers explore this fertile ground for innovation, collaboration within the scientific community will be paramount.
The Future of Cognitive Phenomena Studies
Looking ahead, the integration of innovative techniques such as EPSILON into the study of cognitive phenomena promises to transform our understanding of memory processes. By meticulously mapping the synaptic behaviors that occur during different types of learning experiences, researchers can begin to unravel the complexities of memory formation in a more comprehensive manner. This understanding is crucial for elucidating the differential patterns of synaptic plasticity that accompany various memory types.
Moreover, as the scientific community continues to share resources and insights, the potential for breakthroughs in our comprehension of learning and memory is enormous. Exploring how various neural mechanisms interact and contribute to the overall cognitive experience will not only advance academic knowledge but also enhance strategies for addressing cognitive decline. The future of neuroscience is bright, with the promise of new revelations that can considerably impact therapy and intervention for memory-related disorders.
Exploring the Link Between Memory and Learning
Investigating the link between memory and learning has long been a focus of psychological and neuroscientific research. The dynamic nature of memory formation—rooted in the concept of synaptic plasticity—illustrates how experiences shape our cognitive well-being. This relationship is critical in understanding how information is processed, retained, and recalled.
By deciphering the complexities behind how memories are formed, researchers gain insights into the underlying mechanisms that disrupt cognitive processes in conditions such as dementia. Modern techniques like EPSILON are crucial in this exploration, allowing for a closer examination of the synapses involved in memory retrieval and encoding.
The Importance of Support in Scientific Discovery
Support for scientific discovery plays a vital role in translating basic research into meaningful applications. Adam Cohen highlights the historical trajectory of scientific tools, illustrating that even breakthroughs in unrelated fields can eventually facilitate advancements in health care. Recognizing the importance of sustaining research funding and infrastructural support is essential for fostering the next generation of innovations.
The collaborative effort seen in institutions like Harvard serves as a model for how interdisciplinary approaches can yield groundbreaking results. By uniting chemists, biologists, and neuroscientists, research efforts can be significantly amplified, ultimately enhancing our understanding of complex issues such as dementia and other cognitive impairments. As the field evolves, continued investment in scientific research is key to unlocking future advancements.
Frequently Asked Questions
What is health tracking in relation to learning and memory formation?
Health tracking in relation to learning and memory formation involves monitoring how lifestyle factors impact brain function and memory retention. Techniques in neuroscience research, such as studying synaptic plasticity, provide insights into how dietary choices, exercise, and sleep patterns affect cognitive health and memory formation.
How can health tracking assist in dementia treatment?
Health tracking can play a crucial role in dementia treatment by allowing caregivers and healthcare professionals to monitor cognitive changes and implement early interventions. Using advanced techniques like EPSILON, researchers can identify synaptic changes related to memory, thereby guiding therapies aimed at improving synaptic plasticity and cognitive function in dementia patients.
What role does synaptic plasticity play in effective health tracking?
Synaptic plasticity is pivotal for health tracking as it underlies the brain’s ability to adapt and learn. By observing synaptic changes through health tracking methods, researchers can better understand how different factors influence the brain’s adaptive processes, leading to improved strategies for enhancing memory and preventing cognitive decline.
What advancements does the EPSILON technique bring to memory research in health tracking?
The EPSILON technique significantly advances memory research in health tracking by providing detailed mapping of synaptic behavior. This allows for the observation of how proteins involved in synaptic plasticity respond to various stimuli, offering a clearer picture of the biological underpinnings of memory and leading to potential treatments for cognitive impairments.
How does monitoring neuronal health contribute to understanding memory formation?
Monitoring neuronal health contributes to understanding memory formation by revealing how different variables affect synaptic plasticity—the core mechanism of memory storage. By health tracking neuronal changes, researchers can identify the factors that support or impair learning processes, potentially informing new therapies for neurological disorders like dementia.
Can health tracking impact neuroscience research outcomes?
Yes, health tracking can profoundly impact neuroscience research outcomes by providing valuable data on how lifestyle factors influence brain function. This empirical evidence can inform studies on synaptic plasticity and memory formation, leading to enhanced understanding and treatment options for conditions that affect cognitive health.
What is the importance of synaptic behavior in health tracking studies?
Synaptic behavior is vital in health tracking studies as it directly correlates with how memories are formed and retrieved. By studying synaptic plasticity through methods like EPSILON, researchers can understand the underlying mechanisms of learning and memory, which is essential for developing targeted interventions in cognitive health.
| Key Points | Details |
|---|---|
| New Technique: EPSILON | Maps molecular foundations of learning and memory. |
| Significance | Offers potential new therapies for disorders like dementia. |
| Research Method | Combines fluorescent labeling with advanced microscopy to observe synaptic behavior. |
| AMPARs Role | Key proteins in synaptic plasticity that facilitate learning and memory formation. |
| Findings on Memory Dynamics | Revealed rules for how synapses strengthen or weaken during memory formation. |
| Future Applications | EPSILON will be used across labs worldwide to study cognitive processes. |
Summary
Health Tracking techniques like the newly developed EPSILON method are crucial for understanding the formation of memories and learning processes in the brain. This innovative approach not only sheds light on the molecular mechanisms involved but also holds promise for developing therapies for neurological disorders such as dementia. As researchers continue to explore the dynamics of synaptic plasticity and its implications for memory, it is evident that advancements in health tracking are pivotal in paving the way for improved understanding and treatment of cognitive impairments.
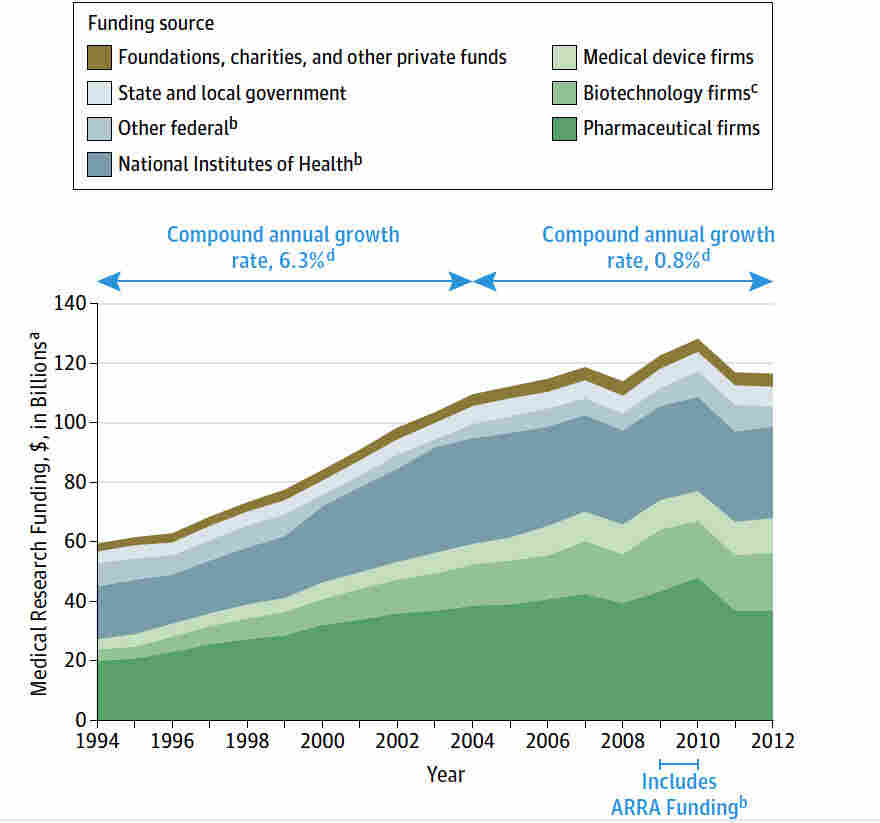
Medical Research Funding: Protecting Patients’ Safety
Medical research funding plays a crucial role in advancing healthcare and ensuring patient safety. Without adequate financial support, the vital processes of clinical research oversight can falter, jeopardizing the wellbeing of participants enrolled in studies. For instance, cuts to federal research grants can severely affect institutional review boards (IRBs), which are responsible for ethical oversight in research. The implications of this disruption can cascade, impacting not only current studies but also eroding public trust in medical research as a whole. Consequently, safeguarding the rights and safety of patients hinges on the consistent allocation of funding to support collaborative and ethical research practices.
Funding for medical research encompasses various forms of financial support aimed at enhancing clinical trials and safeguarding patient welfare. This crucial financial backing facilitates the ethical oversight necessary for research that involves human participants, ensuring that their rights are respected throughout the study process. The interruption of research funding, particularly from federal sources, can lead to significant setbacks in ongoing trials and collaborative efforts across healthcare institutions. Such disruptions not only hinder scientific progress but can also foster distrust among the public, who rely on these studies for innovative treatments and therapies. Ultimately, maintaining steady support for research financing is essential for protecting patient safety and promoting effective oversight in clinical research.
The Importance of Medical Research Funding
Medical research funding is crucial for advancing scientific knowledge and improving healthcare outcomes. It supports the studies that enable researchers to develop new treatments, medications, and technologies that can significantly enhance patient care. Without sufficient funding, institutions may struggle to conduct vital studies that test the effectiveness and safety of new medical approaches, which can lead to stalled innovation in the healthcare industry.
Additionally, federal research grants are essential because they provide the resources necessary for institutions to maintain rigorous ethical oversight. This oversight, often facilitated by Institutional Review Boards (IRBs), ensures that research complies with ethical standards that protect participant safety and rights. A disruption in funding can compromise these safeguards, potentially leading to oversights in how clinical research is conducted.
Frequently Asked Questions
How does medical research funding impact patient safety in clinical trials?
Medical research funding is crucial for ensuring patient safety in clinical trials. It provides necessary resources for institutional review boards (IRBs) to review protocols, monitor participant welfare, and enforce ethical standards. With adequate funding, IRBs can effectively oversee the research process, minimizing risks and protecting the rights and safety of participants.
What is the role of federal research grants in clinical research oversight?
Federal research grants play a vital role in clinical research oversight by providing the financial support needed for developing robust regulatory frameworks. These grants enable institutions to establish and maintain IRB processes that ensure compliance with federal regulations, thereby safeguarding the well-being of research participants.
Can you explain the impact of IRB funding on ethical oversight in research?
IRB funding significantly enhances ethical oversight in research by ensuring that adequate resources are available for thorough reviews of research proposals. With sufficient funding, IRBs can adequately assess risks, ensure informed consent, and uphold ethical standards, ultimately protecting participant safety and maintaining public trust in medical research.
What challenges do researchers face due to cuts in medical research funding?
Cuts in medical research funding severely hinder researchers’ ability to conduct safe and ethical studies. Reduced funding leads to fewer resources for IRBs, resulting in compromised clinical research oversight and increased risks to participant safety. Additionally, ongoing studies may be interrupted, leading to a potential loss of valuable data and diminished public trust in research.
How do federal research grants influence the effectiveness of collaborative research efforts?
Federal research grants are essential for fostering collaborative research efforts, as they provide the necessary funds to streamline processes like single IRB reviews across institutions. By funding multi-site studies, these grants enable researchers to work more efficiently together, ensuring that patient safety and ethical standards are consistently upheld throughout the research process.
What are the implications of insufficient funding on patient safety in medical research?
Insufficient funding for medical research leads to delays in clinical trials, inadequate oversight by IRBs, and potential risks to patient safety. Without adequate resources, researchers may struggle to ensure rigorous ethical standards and comprehensive monitoring, which could ultimately compromise the welfare of participants and the integrity of research outcomes.
How does the SMART IRB system relate to medical research funding?
The SMART IRB system, designed to facilitate oversight for multi-site studies, relies heavily on medical research funding for its operation. This funding allows IRBs to collaborate effectively and streamline approval processes, thus enhancing patient safety and improving the overall efficiency of clinical research.
In what ways can research funding cuts affect patient enrollment in clinical studies?
Research funding cuts can adversely affect patient enrollment in clinical studies by halting ongoing research and preventing new trials from starting. Without adequate resources for outreach and community engagement, potential participants may be unaware of available studies, limiting their access to innovative treatments and undermining the progress of medical research.
What systemic issues arise from a halt in medical research funding?
A halt in medical research funding can create systemic issues such as delayed studies, interrupted patient recruitment, and a decline in the quality of research oversight. This can adversely affect the integrity of clinical findings and contribute to public mistrust of the medical research enterprise, ultimately compromising patient safety.
| Key Point | Description |
|---|---|
| Funding Freeze | The Trump administration’s freeze on over $2 billion in federal research grants disrupted medical research efforts, affecting the rights and safety of patients. |
| SMART IRB System | Administered by Harvard Catalyst, the SMART IRB system is essential for oversight of collaborative medical research across multiple sites. |
| IRB Responsibilities | Institutional Review Boards (IRBs) ensure patient safety and rights by reviewing research proposals and maintaining ethical standards. |
| Impact of Funding Cuts | Cuts to funding halt ongoing studies, delay research, and risk participant safety, further eroding public trust in medical research. |
Summary
Medical research funding is crucial for upholding the safety and rights of patients in research studies. The halt in funding has led to significant disruptions in oversight and has jeopardized the ethical review processes necessary to protect participants. The loss of vital resources like the SMART IRB system directly impacts the ability to conduct thorough and responsible research. Continuing this funding is imperative, as it ensures the integrity of clinical studies and fosters public confidence in the medical research community.

Vaping Cessation Pill: FDA-Approved Aid for Teens
Vaping cessation pill, specifically varenicline, is emerging as a beacon of hope for teenagers and young adults seeking to escape the grips of nicotine addiction. Recent research from Mass General Brigham has revealed that those who use this FDA-approved smoking cessation medication are over three times more likely to successfully quit vaping compared to individuals relying solely on behavioral counseling. With the alarming rates of vaping among teens—approximately 8% of high schoolers vaped in 2024—this treatment offers a scientifically-backed solution to address a pressing public health concern. The effectiveness of varenicline presents a unique opportunity for nicotine addiction treatment, emphasizing the need for accessible options that aid adolescents in quitting. Understanding the implications of such findings is crucial as society seeks to combat the increasing prevalence of vaping among youth.
The vaping discontinuation pill, a term capturing the essence of varenicline, is gaining recognition as an essential component in the fight against nicotine dependency in youth. This newly-highlighted medication, designed for those struggling to stop vaping, promises significant benefits, especially for those aged 16 to 25. With nicotine products becoming increasingly prevalent among young people, a focused approach, including FDA-endorsed smoking cessation solutions, is vital. Innovative strategies and comprehensive treatment methods are being developed to provide effective resources for teens and young adults grappling with the pervasive challenge of vaping. Such advancements illuminate the path towards a healthier future, free from nicotine addiction.
Understanding Varenicline for Teens
Varenicline is an FDA-approved smoking cessation pill that has shown significant promise in helping individuals, particularly teens and young adults, to quit vaping. In recent studies, like the one conducted at Mass General Brigham, it was found that young people aged 16 to 25 who used varenicline were over three times more likely to successfully quit vaping compared to those using placebos. This statistic highlights the potential of designated medical support in combating nicotine addiction within this vulnerable demographic, addressing their specific needs and circumstances.
The effectiveness of varenicline stems from its dual action on nicotine receptors in the brain, which not only reduces the pleasure derived from smoking but also mitigates withdrawal symptoms. For many teens and young adults grappling with nicotine addiction, this can make the transition away from vaping more bearable. As vaping continues to rise among youth, particularly with ease of access and use, incorporating varenicline into cessation programs becomes crucial in addressing the public health crisis associated with nicotine dependency.
The Role of Behavioral Counseling in Vaping Cessation
Although varenicline provides a powerful tool for quitting vaping, behavioral counseling remains an essential component of a comprehensive cessation strategy. The study from Mass General Brigham included weekly behavioral counseling alongside the medication, which demonstrated improved results compared to using varenicline alone. By providing emotional support and practical coping strategies, counselors can help adolescents navigate cravings and triggers related to nicotine addiction, emphasizing that quitting is not just about the physical need but also about changing habits and social behaviors.
Behavioral counseling can aid in reinforcing the motivation to quit and develop resilience against relapse. In the study, participants who were engaged in counseling had higher success rates, showing that medication and psychological support work synergistically to combat the addiction. For teens and young adults, integrating counseling with medication like varenicline represents a holistic approach to overcoming vaping, highlighting the need for multifaceted strategies within nicotine addiction treatment.
The Impact of Vaping on Adolescent Health and Development
Vaping has rapidly become a significant health concern among adolescents, with studies revealing alarming rates of use among high schoolers and young adults. The findings from Mass General Brigham indicate that approximately 8 percent of high school students engaged with vaping in 2024, pointing to a pervasive trend that can jeopardize the health and development of young people. The high levels of nicotine present in many vape products not only foster immediate addiction but also set the stage for greater susceptibility to substance abuse disorders later in life.
Early exposure to nicotine can affect brain development, leading to cognitive impairments and increased vulnerability to addiction. Given that teens are generally more affected by the addictive properties of nicotine, effective cessation methods like varenicline are essential in redirecting their health trajectory. Addressing adolescent vaping through targeted public health strategies that encompass both cessation pills and counseling can pave the way for healthier futures and contribute to reducing broader societal health issues.
The Efficacy of FDA-approved Smoking Cessation Methods
The FDA’s approval of varenicline as a smoking cessation method highlights its credibility and effectiveness in treating nicotine addiction, particularly among younger populations. In clinical trials, such as those reported by Mass General Brigham, varenicline’s ability to facilitate a successful quit rate shows significant promise, with 51 percent of young participants quitting vaping after 12 weeks of treatment. This statistic reinforces the importance of utilizing FDA-approved medications in tobacco cessation programs for youth, aligning with efforts to reduce nicotine addiction in a generation increasingly affected by vaping.
Additionally, the study’s focus on the younger demographic reveals that there is an urgent need for effective nicotine addiction treatment targeted at teenagers. Current cessation methods must adapt to the unique challenges faced by adolescents, such as peer influence and the social acceptance of vaping. Importantly, varenicline’s designation for use in this age group is a step towards addressing these challenges and providing the necessary resources to combat the vaping epidemic.
Combating Teens and Vaping: A Public Health Challenge
Combating the rising trend of vaping among teenagers poses a challenging public health dilemma. The study findings from Mass General Brigham shed light on the urgent need for effective interventions, as vaping is increasingly normalized among youth. With approximately one-quarter of the 18 to 25 age group reported vaped in recent years, understanding the associated health risks and developing appropriate cessation strategies is paramount for public health officials and caregivers alike.
Solutions must encompass educational efforts that highlight the dangers of vaping while simultaneously increasing accessibility to cessation tools like varenicline. Collaborative strategies involving schools, healthcare providers, and community organizations can create an inclusive framework to address vaping among teens. By providing comprehensive support and proven therapeutic options, society can help mitigate the risks of nicotine addiction and promote healthier lifestyles for future generations.
Exploring New Therapeutic Approaches Beyond Varenicline
While varenicline represents a significant advancement in nicotine addiction treatment, ongoing research is crucial to explore additional therapeutic approaches that might benefit adolescents. The Mass General Brigham study emphasizes the need for continuous evaluation of treatment efficacy, particularly as vaping trends evolve. Future investigations might include examining combinations of medications or alternative therapy options that cater to the diverse experiences of youth nicotine users.
By diversifying treatment approaches, there can be a greater opportunity to assist varied demographics, addressing unique challenges faced by different groups, and improving quit rates overall. The goal should remain focused on enhancing the health of young populations, ensuring they have comprehensive access to all available cessation resources and support systems.
The Importance of Support Systems in Quitting Vaping
Support systems play a pivotal role in helping young people quit vaping effectively. A combination of pharmacotherapy like varenicline, alongside robust emotional and social support, can significantly enhance the likelihood of successful cessation. As the study illustrates, participants who received both medication and behavioral counseling reported higher quit rates, showcasing the essence of holistic approaches in nicotine addiction treatment.
Engaging friends, family, and community resources also provides a strong foundation for teens struggling with nicotine dependence. Social networks can serve as motivators and accountability partners, helping adolescents remain committed to quitting. By fostering supportive environments, cessation efforts can lead to better outcomes and provide young individuals with the confidence they need to break free from vaping.
Health Risks Associated with Vaping: A Comprehensive Overview
The health risks tied to vaping cannot be understated, especially for teenagers whose bodies and brains are still developing. Recent studies indicate that vaping can lead to various complications, including respiratory issues, cardiovascular problems, and nicotine addiction. Adolescents who start vaping are more likely to move to combustible tobacco products, further heightening their health risks. Understanding these dangers is crucial in informing cessation methods and public health campaigns.
Given the prevalent use of vapes among youth, educational campaigns must effectively communicate the long-term consequences of nicotine use. With studies backing treatment methods like varenicline, efforts to reduce youth vaping through consistent health risk education paired with access to cessation resources can significantly influence the landscape of adolescent health.
Key Findings from Recent Studies on Vaping and Youth
Recent studies, including the one conducted by researchers at Mass General Brigham, emphasize the critical role of medication in aiding teens and young adults to quit vaping. The significant success rates observed among participants using varenicline indicate a pressing need for healthcare professionals to consider pharmaceutical options when developing treatment plans for young individuals. Findings from this research underline the potential for breaking the cycle of nicotine addiction in youth.
Moreover, these studies advocate for continuous research to adapt to the evolving nature of vaping products and their effects on adolescents. By building upon current findings, further improvements can be made to develop tailored strategies that resonate with younger populations, ensuring lasting impact and support. As the urgency to stop youth vaping escalates, apportioning more resources towards research will be beneficial in effectively mitigating the prevalence of nicotine addiction among teens.
Frequently Asked Questions
What is the vaping cessation pill varenicline and how does it help teens quit vaping?
Varenicline is an FDA-approved smoking cessation pill that is effective in helping teens and young adults quit vaping. Clinical studies show that those aged 16 to 25 taking varenicline are three times more likely to successfully stop vaping than those receiving only behavioral counseling, making it an essential option for nicotine addiction treatment.
Can teens safely use the vaping cessation pill varenicline to quit vaping?
Yes, varenicline is deemed safe for teens aged 16 to 25 who wish to quit vaping. It has been shown not only to be effective but also to prevent a transition to cigarette smoking among those who stop vaping.
How does varenicline compare to behavioral counseling in vaping cessation for young adults?
Varenicline significantly outperforms behavioral counseling alone in aiding vaping cessation among young adults. In clinical trials, 51% of participants using varenicline successfully quit vaping at the 12-week mark, compared to just 14% for those using a placebo with counseling.
What are the potential side effects or concerns associated with using varenicline for quitting vaping?
While varenicline is generally safe, some users may experience mild side effects, such as nausea or insomnia. However, no participants in the studies transitioned to cigarette smoking after quitting vaping, suggesting that its benefits in nicotine addiction treatment outweigh potential risks.
Is the vaping cessation pill varenicline recommended for young adults struggling with nicotine addiction?
Absolutely. Varenicline is an FDA-approved option specifically designed for smoking cessation in adults but is effective for young adults aged 16 to 25 trying to quit vaping, offering a medically-backed approach to combat nicotine addiction.
How does the text support service ‘This is Quitting’ complement varenicline for quitting vaping?
The ‘This is Quitting’ text support service provides additional behavioral support, which, when combined with varenicline, enhances the likelihood of successfully quitting vaping. Participants reported better outcomes when they utilized both the medication and the supportive text service.
What statistics highlight the effectiveness of varenicline in helping teens quit vaping?
Statistics from a recent clinical trial show that at 12 weeks, 51% of teens and young adults taking varenicline successfully stopped vaping, compared to only 14% of those on placebo and 6% using the text-only service. This illustrates the significant impact of varenicline in vaping cessation.
Are there other therapeutic approaches being considered for vaping cessation besides varenicline?
Further research is ongoing to explore additional therapeutic approaches for vaping cessation, targeting younger populations and evaluating different medications alongside behavioral therapies to address the nicotine addiction crisis among teens.
| Key Points |
|---|
| Varenicline is an FDA-approved smoking cessation pill that helps teens and young adults quit vaping effectively. |
| A clinical trial found that participants aged 16-25 taking varenicline were three times more likely to quit vaping than those on placebo. |
| The study involved 261 participants and used behavioral counseling along with the medication for comprehensive support. |
| After 12 weeks, 51% of varenicline users had quit vaping compared to only 14% of placebo users. |
| The findings underline the importance of medication for helping young people break free from nicotine addiction. |
| Varenicline was found to be safe; no participants who quit vaping switched to cigarettes after treatment. |
Summary
The vaping cessation pill, varenicline, has proven to be an effective solution for helping young individuals cease their vaping habits. This FDA-approved medication significantly increases the chances of quitting among teens and young adults, making it a valuable resource in the fight against nicotine addiction. With further research and expanded treatment options, there is hope for more effective strategies to assist this vulnerable population in overcoming their dependence on vaping.

Federal Research Grants: A Lifeline for Health Studies
Federal research grants play a crucial role in advancing public health research, providing essential funding for health studies aimed at addressing critical issues like cancer risk. With the National Institutes of Health (NIH) grant application process serving as a benchmark for scientific research funding, these grants empower researchers to explore innovative strategies that can significantly enhance community health outcomes. For instance, scholars such as Karen Emmons and Jorge Chavarro rely on these grants to explore novel approaches in their respective fields, making a profound difference in human life. However, the landscape of federal research funding can be complex, often accompanied by challenges that can hinder promising studies. Despite these obstacles, the significance of securing funding for health studies cannot be understated, as it ultimately enables researchers to confront pressing health crises and contribute to valuable scientific knowledge.
In the realm of scientific inquiry, federal funding initiatives emerge as fundamental pillars for researchers striving to make a difference in public health. Variously known as government research grants or public funding for scientific endeavors, these financial resources enable scholars to embark on groundbreaking projects, particularly in areas like cancer prevention and health interventions. The intricate NIH grant process demonstrates how rigorously evaluated proposals can lead to transformative research outcomes, bolstering the scientific community’s efforts to address health disparities. Researchers dedicated to health studies, such as those focusing on nutrition and reproductive health, navigate a competitive landscape that underscores the importance of funding for innovative investigations. Ultimately, the partnership between government agencies and academic institutions underscores a commitment to advancing knowledge that supports healthier communities.
The Importance of Federal Research Grants in Public Health
Federal research grants play a pivotal role in advancing public health initiatives. For researchers like Karen Emmons at the Harvard T.H. Chan School of Public Health, these grants are not merely financial lifelines; they represent the validation of an idea capable of making a positive impact on society. Secure funding allows researchers to investigate innovative methods to tackle issues such as cancer risk, which disproportionately affects under-resourced communities. Without these grants, many promising studies could be silenced, leaving critical health challenges unaddressed.
Furthermore, the competition for federal research grants pushes scientists to refine their ideas and methodologies continually. The rigorous National Institutes of Health (NIH) application process ensures only the most compelling proposals receive funding. This scrutiny mandates that researchers provide a detailed plan that includes existing research gaps and methodological soundness. As Jorge Chavarro notes, this is not just about having an idea—it’s about demonstrating the ability to execute that idea effectively. This emphasis on detailed planning cultivates a research environment that advances public health and fosters innovation.
Navigating the NIH Grant Application Process
Navigating the NIH grant application process can seem daunting due to its complexity and competitiveness. Applicants must start with a precise and compelling one-page statement known as the specific aims page, highlighting how their study aims to fill significant gaps in existing research. Following this, the full application can exceed 100 pages, demanding thorough documentation of preliminary findings and detailed methodologies. The comprehensive nature of this process is designed to ensure only the most promising health studies receive federal funding, reflecting the importance of quality and relevance in scientific research.
Moreover, successful grant applications are not purely about the science—ethics and community engagement also play critical roles. Researchers like Emmons emphasize the need for ethical treatment of human participants throughout their studies, which adds another layer of complication to the process. Budgetary constraints further challenge applicants, making it crucial for them to justify each expense meticulously. The need for such detailed proposals illustrates the commitment to ensuring that public funds are used responsibly and effectively, reinforcing trust in the research funding process.
Challenges Faced by Public Health Researchers
Despite the vital contributions of public health research to society, researchers often face substantial challenges in securing funding. The recent halt of over $2.2 billion in research grants to Harvard has highlighted the precarious nature of federal funding and the impact of political decisions on scientific endeavors. For many researchers, disruptions like these can delay critical studies focused on diseases such as tuberculosis and neurodegenerative disorders. Emmons and Chavarro’s experiences underscore the fragility of funding streams and how they directly impact the ability to carry out important health studies.
Additionally, researchers are often required to balance the need for innovative research with the limitations of their budgets. With the cost of conducting valid, large-scale studies on the rise, scientists are pressured to find efficient ways to allocate limited resources. This includes justifying expenses for necessary equipment or materials, as even small purchases can influence the overall success of a grant application. Such pressures can stifle creativity and discourage compromise, making it harder for researchers to pursue groundbreaking investigations that may lead to significant public health advancements.
The Role of Networking and Collaboration in Research
Networking and collaboration are essential components of successful public health research and securing federal grants. Researchers must build strong relationships with community partners and fellow scientists to foster a supportive environment for their studies. As Emmons notes, knowing what other researchers are working on helps avoid redundancy and encourages innovative approaches. Collaborations can also strengthen grant applications, as they demonstrate a network of support and diverse expertise in addressing complex health challenges.
Interdisciplinary collaborations, in particular, open the door to new insights and methodologies that can elevate a research proposal’s potential impact. Chavarro’s experience in nutritional epidemiology illustrates how linking various research fields can lead to groundbreaking findings. By engaging with colleagues from different areas, researchers can combine perspectives, enhancing their studies’ relevance and effectiveness. Such collaborative approaches not only strengthen proposals but also foster a culture of inclusivity and shared knowledge that benefits the entire scientific community.
How Research Funding Shapes Public Health Policies
The connection between research funding and public health policy is undeniable. Successful studies backed by federal research grants often translate into evidence-based practices that inform health policies. For instance, Emmons’ work on cancer risk strategies can shift public health approaches, advocating for interventions designed to better serve under-resourced communities. This cyclical relationship underscores the necessity of sustained funding in enabling researchers to provide actionable insights that shape the health landscape.
In response to significant findings borne from federally funded research, policymakers can implement reforms that directly improve health outcomes. This linkage between evidence, funding, and policy is critical for advancing public health initiatives that lead to enhanced community well-being. Consequently, ensuring that robust funding mechanisms remain in place is essential for nurturing future research endeavors, empowering scientists to explore innovative solutions to pressing health issues.
The Necessity of Ethical Standards in Health Research
Ethics play a central role in the conduct of public health research, particularly when human participants are involved. Researchers must adhere to strict ethical guidelines to ensure the safety and dignity of individuals taking part in studies. In the context of securing federal funding, demonstrating a commitment to ethical standards is paramount, as it can significantly influence the success of grant applications. Emmons emphasizes the importance of treating participants ethically and transparently, as these principles are fundamental to building trust with the community and obtaining approval from funding agencies.
Moreover, ethical considerations extend beyond the treatment of subjects; they also pertain to the overall integrity of the research process. Researchers must ensure their methodologies are sound and that their findings are reported accurately. Failure to adhere to ethical standards can result in lasting damage not only to individual careers but also to the credibility of the broader scientific community. Upholding these standards is essential, particularly in public health research, where the ultimate goal is to improve health outcomes and deliver equitable interventions.
The Future of Health Studies: Trends and Innovations
The future of health studies is poised for transformation with the emergence of new technologies and methodologies that enhance the research landscape. With advancements in data analytics and health informatics, researchers are now able to access and analyze vast datasets that provide insights into health trends and challenges. This evolution presents new opportunities for public health researchers to explore uncharted territories, advancing fields like cancer risk research in ways previously thought impossible.
Moreover, as funding for health studies grows more competitive, there is an increasing emphasis on innovative approaches that can demonstrate tangible benefits to public health. Researchers are encouraged to think outside the box and leverage interdisciplinary methods, combining insights from life sciences, social sciences, and technology. The shift towards collaborative research that encompasses various specialties will be crucial in addressing complex health issues, ultimately fostering a more comprehensive understanding of factors influencing public health outcomes.
The Impact of Political Climate on Research Funding
Research funding is inherently influenced by the political climate, which can lead to significant fluctuations in available federal resources. Recent actions, such as the freezing of $2.2 billion in grants, illustrate how political decisions can directly impact public health research initiatives. This instability creates uncertainty for researchers, as they navigate an environment where grant opportunities may suddenly be curtailed or redirected based on changing administrations and policies.
Moreover, the prioritization of certain health research areas over others can reflect broader political agendas, potentially skewing the focus of scientific inquiry. Emmons and Chavarro’s experiences highlight how external pressures can disrupt long-term research plans, necessitating adaptability from scientists. As researchers advocate for the importance of their work in advancing public health, they must remain vigilant to ensure that funding aligns with critical health needs, regardless of the prevailing political landscape.
The Challenges of Justifying Research Budgets
Justifying budgets is one of the crucial steps in the grant application process, particularly for public health researchers who must navigate limited funding resources. As emphasized by Chavarro, every line item in a research budget requires careful explanation, ensuring that expenses are deemed necessary for successful project execution. This detailed justification is not only a reflection of resourcefulness but also essential for establishing the credibility of the research proposal.
Budgeting challenges often extend to demonstrating the value of specific items, such as equipment or personnel resources. Researchers must clearly articulate why these expenditures are vital for innovative research and how they contribute to advancing the project’s overall objectives. The burden of justifying costs can be stressful for scientists, but it also cultivates a mindset of accountability and thoughtful resource allocation, which is crucial for the sustainability of public health research initiatives.
Frequently Asked Questions
What are federal research grants and how do they impact public health research?
Federal research grants are financial awards provided by government agencies, such as the NIH, to support scientific research initiatives. These grants are crucial for public health research as they fund projects aimed at improving health outcomes, tackling diseases, and conducting vital studies like cancer risk research. Researchers rely on these grants to secure the financial resources needed to conduct innovative and impactful studies.
How can I apply for funding for health studies through federal research grants?
To apply for funding for health studies via federal research grants, you must follow the NIH grant application process, which includes developing a research proposal, outlining specific aims, and detailing methodologies. Your research should address significant public health concerns and demonstrate innovation. Applications are rigorously peer-reviewed, and only the best proposals receive funding.
What is the NIH grant application process for scientific research funding?
The NIH grant application process for scientific research funding involves multiple steps: first, developing a compelling research proposal that includes specific aims and a detailed methodology. Then, submit the full application, typically exceeding 100 pages, including supporting documents. After submission, your application will undergo reviews by Scientific Review Groups to evaluate its scientific merit, innovation, and alignment with public health needs.
What do I need to include in my NIH grant application for cancer risk research?
When applying for an NIH grant for cancer risk research, your application should include a clear statement of specific aims addressing existing research gaps, a comprehensive literature review, methodologies, preliminary data, ethical considerations, and a well-justified budget. Be prepared to demonstrate how your study will advance knowledge in cancer prevention or treatment.
What challenges do researchers face in securing federal research grants for public health studies?
Researchers encounter several challenges when securing federal research grants for public health studies, including intense competition due to limited funding, the need for thorough preparation and collaboration, navigating complex application processes, and adhering to stringent ethical guidelines. Additionally, fluctuations in government support can impact the availability of resources for public health research.
How do federal research grants support cancer risk research initiatives?
Federal research grants play a vital role in supporting cancer risk research initiatives by providing funding for studies aimed at understanding cancer causes, prevention, and treatment. These grants enable researchers to conduct large-scale epidemiological studies, develop public health interventions, and advance scientific knowledge, ultimately aiming to reduce cancer incidence and improve patient outcomes.
What is the success rate for NIH grants in scientific research funding?
The success rate for NIH grants in scientific research funding varies by grant type and institute. For instance, in 2023, the National Cancer Institute reported a success rate of 14.6 percent for R01 grants. This implies that only a fraction of submitted proposals will be awarded funding, highlighting the competitive nature of the grant application process.
What role do community partnerships play in securing federal research grants for health studies?
Community partnerships are essential for securing federal research grants for health studies as they provide valuable insights into local health needs, enhance the feasibility of research projects, and ensure that studies are grounded in real-world contexts. Collaborating with community organizations can strengthen grant proposals by demonstrating the relevance and potential impact of research initiatives.
| Key Point | Description |
|---|---|
| Significance of Federal Grants | Obtaining federal research grants is crucial for public health researchers as it enables them to conduct impactful studies that can help improve human health. |
| Challenges in the Grant Process | The federal grant process can be competitive and time-consuming, requiring extensive preparation and justification for funding requests. |
| Impact of Political Decisions | Recent actions by the Trump administration freezing funding have threatened ongoing research projects at institutions like Harvard. |
| Ethical Considerations | Research involving human participants mandates stringent ethical guidelines and rigorous review processes to protect subjects. |
| Funding Success Rates | The likelihood of receiving funding is low, with success rates for major grant types like R01 being as low as 14.6%. |
| Long-term Partnerships | The relationship between public and private sectors is vital for advancing science and improving public health outcomes. |
Summary
Federal research grants play a pivotal role in advancing public health initiatives and supporting researchers like Karen Emmons and Jorge Chavarro, who strive to reduce diseases and improve nutrition. Despite political challenges that have threatened funding, the ongoing commitment to securing these grants remains essential for driving innovation and improving health outcomes. The meticulous grant application process ensures that only the most promising research proposals receive funding, demonstrating the importance of federal support in scientific research.

Bile Imbalance and Liver Cancer: Key Findings Revealed
Bile imbalance and liver cancer have become critical topics in understanding liver health, particularly in the context of hepatocellular carcinoma (HCC), the most prevalent type of liver cancer. Recent research highlights the crucial role bile acids play, as they not only aid in fat digestion but also influence various metabolic processes that can lead to serious liver disease. Disruption of bile acid regulation has been identified as a significant risk factor for liver injury and inflammation, paving the way for cancer development. The YAP-FXR relationship emerges as a pivotal molecular switch, demonstrating how YAP’s repressive action on the FXR receptor can trigger liver cancer progression. With insights from studies on liver cancer causes, these developments offer new avenues for liver disease treatment, promising improvements in patient outcomes.
When we discuss bile imbalance and liver cancer, we also delve into the broader realm of liver health issues and neoplastic conditions affecting this vital organ. The intricate relationship between bile acids, their metabolism, and hepatic diseases showcases the gravity of hormonal influences on liver function and growth. Hepatocellular carcinoma, marked as a leading cause of cancer-related morbidity, underscores the necessity of understanding the mechanisms that contribute to its emergence, particularly through disrupted bile acid homeostasis. As research uncovers the complex interplay between signaling pathways—especially involving YAP and FXR—the potential for innovative therapeutic approaches arises, demonstrating the need for ongoing exploration in liver disease treatment. By focusing on bile as a crucial factor in liver pathology, we can illuminate the path to better management strategies.
Understanding the Role of Bile Acids in Liver Health
Bile acids are crucial elements produced by the liver, playing an essential role in digestion and metabolism. They facilitate the emulsification of dietary fats, aiding in their absorption in the intestine. Aside from their digestive functions, bile acids also have significant roles in metabolic regulation, affecting how the body processes nutrients and maintains homeostasis. When the balance of bile acids is disrupted—often due to liver disease—this can lead to severe consequences, including inflammation and fibrosis, which may ultimately escalate to hepatocellular carcinoma (HCC), the most common type of liver cancer.
Research has shown that an imbalance in bile acids can trigger liver injury and promote cancer development. For instance, an increase in bile acids can lead to toxic accumulation within liver cells, causing cellular stress and damage. This cascade of events not only contributes to liver disease but also enhances the risk of developing cancers such as hepatocellular carcinoma. Scientists are actively investigating how this bile imbalance may affect the liver’s overall function and its capacity to ward off diseases.
The Connection Between Bile Imbalance and Liver Cancer
The link between bile acid imbalance and liver cancer is becoming increasingly evident in scientific research. One of the pivotal findings is that an excess of bile acids can lead to the activation of oncogenic pathways, such as the Hippo/YAP pathway. Normally, this pathway plays a crucial role in controlling cell growth and tissue development. However, disruptions caused by an imbalance in bile acids can lead to aberrant signaling that promotes the proliferation of cancerous liver cells.
A study revealed that when YAP—a key protein in the Hippo pathway—is overactivated due to bile acid dysregulation, it represses the function of the Farnesoid X receptor (FXR). This dysfunction results in further bile acid accumulation, creating a vicious cycle of inflammation and fibrosis that predisposes the liver to cancer. By restoring the balance of bile acids or enhancing FXR function, there is potential to halt this progression, highlighting the importance of maintaining bile acid homeostasis in preventing liver cancer.
Insights into YAP and FXR: Key Players in Liver Disease
The dynamic between YAP and FXR is critical in understanding liver disease’s underlying mechanisms. YAP is known for its role in promoting tumor growth; however, its interaction with bile acid metabolism presents a complex picture. In healthy liver function, FXR regulates bile acid levels, ensuring they remain within a safe range. But when YAP is activated by bile acid accumulation, it alters FXR’s effectiveness, leading to increased levels of bile acids that can cause further liver damage.
Understanding this relationship opens new doors for liver disease treatment. By focusing on the inhibition of YAP’s repressive function on FXR, researchers could develop therapies that restore the balance of bile acids within the liver. Potential pharmacological interventions may include drugs aimed at enhancing FXR activity or promoting the excretion of excess bile acids, both of which could significantly diminish the risk of liver cancer progression.
Research Advancements in Liver Disease and Cancer
Recent studies have revealed substantial insights into liver disease and cancer, particularly focusing on the roles of bile acids and related signaling pathways. As researchers delve deeper into the mechanisms that govern liver function and its responses to bile acid imbalances, new therapeutic targets are emerging. For instance, treatments that effectively enhance FXR activity may represent a promising avenue for mitigating liver damage and preventing the onset of hepatocellular carcinoma.
These advancements are essential not just for understanding liver cancer causes but also for developing comprehensive treatment plans that target the molecular pathways involved in liver disease. As this research evolves, it may also uncover broader implications for understanding metabolic diseases linked to bile acid dysregulation, marking an exciting frontier in hepatology.
The Future of Liver Cancer Treatment
As researchers continue to elucidate the complexities of liver disease and its connection to bile imbalance, the future of liver cancer treatment looks promising. Strategies that focus on rectifying bile acid dysregulation and targeting specific molecular pathways, like the Hippo/YAP axis, could lead to novel therapeutic interventions. This shift towards personalized medicine, tailored to the intricacies of an individual’s liver metabolism and cancer progression, marks a significant progression in oncological research.
Moreover, innovative pharmacological solutions aimed at enhancing the function of FXR may not only alleviate liver damage but could also serve as a preventative measure against the development of hepatocellular carcinoma. Continuous support from institutions like the National Institutes of Health is vital in driving forward this promising research, ensuring that new insights translate into effective treatments for patients suffering from liver diseases.
Bile Acids: A Double-Edged Sword
While bile acids are essential for digestion and metabolic processes, elevated levels can act as a double-edged sword. On one hand, they are necessary for the absorption of fats and fat-soluble vitamins; on the other hand, an excess can lead to toxicity and liver injury. Understanding this dual role is crucial for developing strategies aimed at balancing bile acid levels, preventing liver disease, and minimizing the risk of liver cancer.
Research has shown that managing bile acid concentrations can significantly impact liver health. By regulating levels through dietary adjustments, pharmacological interventions, or lifestyle changes, individuals may reduce their risk of developing liver-related conditions, including hepatocellular carcinoma. Continued research in this area is fundamental in uncovering the full potential of bile acids as both therapeutic agents and contributors to liver pathology.
Implications of Bile Acid Regulation on Overall Health
The regulation of bile acids extends beyond liver health, influencing overall metabolic functions in the body. Bile acids are involved in the modulation of glucose metabolism, lipid homeostasis, and even inflammation. Therefore, an imbalance in bile acids can cause widespread effects not only on liver function but also on systemic health, emphasizing the need for comprehensive approaches to address bile acid dysregulation.
Recognizing the interplay between bile acids and various bodily systems could pave the way for preventative health strategies. By implementing lifestyle changes that promote bile acid balance—such as diet and exercise—individuals may experience improvements not only in liver health but also in their overall metabolic efficiency. These insights highlight the significance of bile acids in holistic health management.
Innovative Approaches to Bile Management in Liver Disease
As understanding of bile acid roles in liver pathology evolves, so too do innovative approaches to managing bile acid levels in patients with liver disease. Therapies that promote the secretion of bile acids or enhance the liver’s capacity to process them can be cornerstones in treatment paradigms aimed at preventing hepatocellular carcinoma. Such strategies require robust research and clinical trials to assess their effectiveness and safety in diverse patient populations.
Furthermore, adopting a multifaceted treatment approach may yield the best outcomes for individuals with compromised liver function. By combining dietary management, pharmacological interventions, and lifestyle modifications, there is potential not only to treat existing conditions but also to prevent the development of diseases such as liver cancer. Collaborative research efforts are essential to bring these innovative ideas into clinical practice.
Collaboration in Liver Research: A Multidisciplinary Effort
Addressing the complexities of liver diseases and their connection to bile acid dysregulation demands a multidisciplinary research approach. Collaboration between hepatologists, molecular biologists, and pharmacologists is crucial for uncovering the intricate mechanisms that lead to liver cancer. By combining expertise from various fields, more comprehensive and effective treatment strategies can be developed.
Participating in networks such as the Dana-Farber/Harvard Cancer Center allows for the sharing of knowledge and resources, enhancing the research capabilities of each individual entity focused on liver disease. This collaborative spirit will be instrumental in translating research findings into real-world applications, ultimately improving patient outcomes in liver health and cancer management.
Frequently Asked Questions
How is bile imbalance linked to liver cancer?
Bile imbalance occurs when the production and regulation of bile acids are disrupted. This can lead to liver diseases, including hepatocellular carcinoma (HCC), the most common form of liver cancer. When bile acids accumulate in the liver, they contribute to fibrosis, inflammation, and ultimately tumor formation.
What are the causes of liver cancer and how does bile imbalance play a role?
Liver cancer can be caused by various factors, including chronic liver disease, hepatitis infections, and bile imbalance. Disruption in bile acid metabolism affects liver health, leading to conditions like liver inflammation and fibrosis, which can progress to hepatocellular carcinoma (HCC).
What is the relationship between YAP and bile acids in liver cancer?
YAP (Yes-associated protein) is involved in the regulation of bile acid metabolism. The activation of YAP disrupts the function of FXR (Farnesoid X receptor), a vital bile acid sensor. This disruption leads to bile acid overproduction, contributing to liver injuries and increasing the risk of hepatocellular carcinoma (HCC).
What treatments are available for liver disease linked to bile acid imbalance?
Treatment for liver disease caused by bile acid imbalance may involve targeting the FXR pathway or ensuring proper bile acid excretion. Pharmacological interventions that enhance FXR function or inhibit YAP’s repressor function have shown promise in reducing liver damage and curbing cancer progression.
What role do bile acids play in metabolic processes related to liver cancer?
Bile acids function beyond digestion; they also play a hormone-like role in metabolism. Their imbalance can lead to metabolic disruption within the liver, which is a contributing factor in the development of liver diseases and can enhance the risk of hepatocellular carcinoma (HCC).
How does liver disease treatment help prevent hepatocellular carcinoma?
Effective liver disease treatment focuses on correcting bile acid imbalances and enhancing metabolic pathways associated with liver function. By improving liver health and regulating bile production, these treatments can reduce the progression to hepatocellular carcinoma (HCC) and mitigate the effects of liver inflammation and fibrosis.
What are the implications of the Hippo/YAP pathway in liver cancer treatment?
The Hippo/YAP pathway is crucial in liver cancer as it regulates cell growth and bile acid metabolism. By targeting mechanisms within this pathway, such as enhancing FXR activity, researchers hope to develop new treatments that can prevent or halt the progression of hepatocellular carcinoma (HCC) linked to bile imbalances.
Can enhancing FXR function improve outcomes in liver cancer patients?
Yes, enhancing FXR function is considered a promising approach in managing liver diseases related to bile imbalance. By promoting FXR activity, it may be possible to restore bile acid homeostasis, reduce liver inflammation, and prevent the development of hepatocellular carcinoma (HCC).
| Key Points | Details |
|---|---|
| Bile Imbalance and Liver Cancer | A critical imbalance in bile acids can trigger liver diseases including hepatocellular carcinoma (HCC), the most common form of liver cancer. |
| Key Molecular Switch Identified | The study identifies a key molecular switch that regulates bile, potentially offering new treatment avenues for liver cancer. |
| Bile’s Role in Digestion | Bile aids in fat digestion and also regulates metabolic processes due to bile acids acting similar to hormones. |
| Hippo/YAP Pathway | The Hippo/YAP signaling pathway is crucial for cell growth regulation related to cancer. YAP inhibits FXR, a bile acid sensor. |
| Consequences of YAP Activation | YAP activation can lead to bile acid accumulation, fibrosis, inflammation, and progression towards liver cancer. |
| Potential Treatments | Blocking YAP’s repressor activity or enhancing FXR function could mitigate liver damage and cancer progression. |
Summary
Bile imbalance is closely linked to liver cancer, particularly hepatocellular carcinoma (HCC). A newly discovered molecular switch provides important insights into how bile acid regulation affects liver health. By unraveling the complex interactions between bile acids and the YAP signaling pathway, researchers can explore novel treatments that may help prevent liver cancer by restoring bile homeostasis. This discovery holds significant promise for future therapeutic strategies aimed at combating liver disease.

Alzheimer’s Disease Research: Insights from Beth Stevens
Alzheimer’s disease research is at the forefront of a scientific evolution, driven by the groundbreaking investigations of neuroscientist Beth Stevens. Exploring the role of microglial cells, the brain’s immune system, Stevens has revealed their pivotal function in maintaining neural health through processes like synaptic pruning. However, when microglia malfunction, the consequences can be dire, leading to neurodegenerative diseases such as Alzheimer’s and Huntington’s. At the Stevens Lab, significant strides are being made to identify biomarkers and therapeutic strategies that could drastically improve the lives of millions grappling with Alzheimer’s. Through her passion and dedication, Stevens embodies the relentless pursuit of knowledge necessary to unlock the mysteries of the brain and offer hope where there is none.
Investigations into dementia-related illnesses are shaping the landscape of neurological science, particularly in understanding how the brain’s defense mechanisms impact overall cognition. Beth Stevens’ work elucidates the essential role of glial cells—critical components of the brain’s immune response—in managing neural integrity and function. These glial cells are not only instrumental in synapse management but also play a crucial role in thwarting neurodegenerative disorders, emphasizing the intersection of brain health and immune functionality. As researchers continue to explore these complex interactions, the insights garnered from such studies have the potential to revolutionize approaches towards treating cognitive decline. This avenue of research opens up new frontiers in discovering effective strategies to combat the effects of age-related brain deterioration.
Understanding Microglial Cells and Their Role in Neurodegenerative Diseases
Microglial cells are a vital component of the brain’s immune system, acting as sentinels that monitor the health of the central nervous system. In recent years, research has illuminated their critical role in maintaining brain homeostasis and synaptic integrity. When functioning properly, these cells assist in the removal of damaged neurons and facilitate synaptic pruning, a process essential for optimal neural connections. However, when microglial cells become dysfunctional, they can contribute to the progression of neurodegenerative diseases, including Alzheimer’s disease. This aberrant activity can lead to improper synaptic pruning, resulting in cognitive decline and other devastating symptoms.
Current investigations into microglial biology are unveiling connections between these immune cells and various neurodegenerative disorders. As researchers like Beth Stevens demonstrate, understanding how microglia interact with neuronal environments can reveal new targets for therapeutic intervention. By exploring the intricate signaling pathways in which microglia engage, scientists are shifting towards a more nuanced understanding of brain health and disease. Such insights are essential for developing innovative treatments aimed at mitigating the effects of diseases like Alzheimer’s, ultimately improving patient outcomes and transforming care approaches.
The Groundbreaking Research of Beth Stevens on Alzheimer’s Disease
Beth Stevens has made significant contributions to the field of Alzheimer’s disease research through her groundbreaking studies on microglial cells. By focusing on how these immune cells manage synaptic pruning, Stevens and her team have uncovered crucial information that links dysregulated microglial activity to the pathogenesis of Alzheimer’s. Such findings highlight the importance of early intervention strategies that aim to restore normal microglial function before irreversible damage occurs. The implications of her research are vast, potentially paving the way for novel biomarkers that could predict the onset of Alzheimer’s symptoms long before conventional clinical signs manifest.
Her exploration into the brain’s immune system has opened new avenues for effectively tackling neurodegenerative diseases. Stevens emphasizes that supporting foundational research, driven by curiosity, is essential for scientific advancement. The link between microglial dysfunction and diseases such as Alzheimer’s demonstrates how fundamental insights into synaptic biology can lead to practical applications in diagnostics and therapeutics. Through the investigation of microglial behavior, researchers can better understand the complexities of brain disorders, thus enhancing strategies for prevention and treatment for millions impacted by these conditions.
The Importance of Synaptic Pruning in Brain Development and Disease
Synaptic pruning is a biological process essential for the proper development and function of neural circuits in the brain. During early brain development, microglial cells play a critical role in the selective elimination of excess synapses, ensuring that neural connections are optimized for efficient communication. This pruning process is not only vital for healthy brain development but continues to be an important mechanism throughout life, influencing learning, memory, and overall cognitive function. Disruptions in this mechanism have been linked to various neurodegenerative diseases, including Alzheimer’s, highlighting the importance of understanding how synaptic pruning goes awry.
Research has shown that improper synaptic pruning can lead to an imbalance in neural connectivity, which is characteristic of Alzheimer’s disease and other cognitive disorders. By studying microglial activity in relation to synaptic pruning, researchers aim to uncover the underlying etiologies of these disorders. Understanding the delicate balance of neural networking could inform future therapeutic strategies aimed at correcting synaptic abnormalities. It is crucial for the scientific community to continue exploring these processes to not only illuminate the mechanisms of disease but also to foster the development of innovative treatments that can mitigate the impact of neurodegenerative diseases on individuals and society.
Revolutionizing Alzheimer’s Disease Therapeutics Through Misguided Immune Responses
The immune system’s response plays a dual role in neurodegenerative diseases, particularly in the context of Alzheimer’s disease. Misguided immune responses can lead to detrimental effects, exacerbating existing neuroinflammation and contributing to neuronal death. Research led by scientists like Beth Stevens has shown how microglial cells, when activated incorrectly, can result in the over-pruning of synapses, adversely affecting cognitive functions. This relationship between immune dysfunction and neuronal health is a burgeoning area of study, as understanding it could lead to more targeted interventions for Alzheimer’s.
To combat these adverse immune responses, ongoing research is focusing on the mechanisms that govern microglial activity. By elucidating these pathways, scientists hope to identify potential intervention points, allowing for the modulation of microglial behavior to promote neuroprotection and support neuronal health. The goal of this research is not only to improve therapeutic outcomes for those suffering from Alzheimer’s but also to provide a deeper understanding of how neurodegenerative diseases can be approached from an immune system perspective, ultimately reshaping treatment paradigms in neurology.
NIH Support: The Backbone of Alzheimer’s Disease Research
The National Institutes of Health (NIH) has played a pivotal role in advancing Alzheimer’s disease research, providing essential funding to scientists exploring the complexities of neurodegeneration. The support received by researchers like Beth Stevens has enabled the pursuit of critical questions about the brain’s immune system and its relationship to diseases such as Alzheimer’s. NIH funding has facilitated the exploration of exciting new hypotheses and innovative research methodologies, driving forward our understanding of how microglial cells function and contribute to synaptic health.
This commitment to foundational research underscores the importance of sustained investment in understanding the biological underpinnings of Alzheimer’s disease. The findings derived from well-supported studies not only enrich the scientific community’s knowledge but also lead to actionable insights that can translate to therapeutic and diagnostic advancements. With NIH backing, the potential for breakthroughs increases exponentially, offering hope to millions affected by Alzheimer’s disease and other neurodegenerative conditions.
The Intersection of Curiosity-Driven Science and Clinical Applications
Curiosity-driven science lies at the heart of groundbreaking discoveries in the field of Alzheimer’s research, particularly in understanding the role of microglial cells. Beth Stevens’ journey exemplifies how initial explorations into seemingly unrelated areas—like the visual system of mice—can yield valuable insights into complex neurological conditions. By allowing scientists the freedom to follow their research interests, we can uncover significant connections that lead to clinical applications in treating diseases such as Alzheimer’s.
As Stevens points out, the connection between foundational research and clinical outcomes is not always immediately apparent. However, it is this very inquiry-based approach that enables researchers to tackle questions that cannot be addressed in human studies. This innovative mindset fosters a deeper understanding of brain function and disease, paving the way for targeted therapies that aim to improve quality of life for individuals affected by neurodegenerative diseases. Thus, supporting curiosity-driven research will be essential for the future of Alzheimer’s disease intervention.
Innovations in Alzheimer’s Disease Biomarkers and Therapeutics
The advancement of biomarkers in Alzheimer’s disease is crucial for early detection and intervention. As Beth Stevens emphasizes, understanding microglial functioning and its impact on synaptic pruning can provide critical insights into the inflammatory processes underlying the disease. Identifying reliable biomarkers can help in assessing individual risk and potentially directing treatments at the most opportune moments in the disease course. The goal is to transform our current approach from a reactionary stance to one that emphasizes prevention and timely therapeutic intervention.
Recent investigations in Stevens’ lab have the potential to influence the development of novel therapeutics aimed at restoring normal microglial function, preventing the harmful effects of improper synaptic pruning. By focusing research efforts on the identification and application of these biomarkers, scientists can create a roadmap for managing Alzheimer’s disease more effectively. Ultimately, innovation in biomarker research holds the key to enhancing patient care and establishing new standards in how we approach Alzheimer’s disease treatment.
Implications of Neuroinflammation in Alzheimer’s Progression
Neuroinflammation has emerged as a significant contributor to the progression of Alzheimer’s disease, with microglial cells taking center stage in this narrative. Chronic activation of microglia can lead to neurotoxic effects, exacerbating neuronal loss and cognitive decline. Understanding the intricacies of how neuroinflammatory responses are regulated is critical for developing strategies to slow or prevent Alzheimer’s progression. The work of researchers like Beth Stevens expands upon this concept, highlighting the need for a concerted effort to investigate these immune dynamics in the context of neurodegeneration.
This research could lead to innovative therapeutic approaches aimed at modulating microglial behavior to foster a more protective environment for neurons. By targeting neuroinflammatory pathways, scientists can develop drugs that potentially alleviate symptoms and halt disease advancement. This promising area of exploration emphasizes the potential for harnessing the immune system to combat neurological diseases, indicating that a deeper understanding of neuroinflammation may be a gateway to novel Alzheimer’s disease management strategies.
Advancements in Alzheimer’s Education: A Community Perspective
Educating communities about Alzheimer’s disease is essential for managing its impact on individuals and families. Comprehensive education initiatives can demystify the complexities of the disease, fostering understanding and support for those affected. By sharing insights from research, particularly regarding the role of microglial cells and neuroinflammation, communities can become better equipped to engage with and support Alzheimer’s patients. Such educational efforts are critical in building a compassionate society that recognizes the value of medical research and its implications for public health.
Furthermore, community engagement can drive advocacy for continued support of Alzheimer’s disease research. By understanding the science behind neurodegenerative diseases, community members can advocate for necessary funding and resources that facilitate research advancements. The role of institutions like the NIH in providing grants illustrates the importance of public investment in scientific endeavors. As communities rally around the knowledge gained from studies on Alzheimer’s, they create an environment ripe for collaboration that ultimately leads to a better understanding of, and solutions for, the challenges posed by neurodegenerative diseases.
Frequently Asked Questions
How do microglial cells contribute to Alzheimer’s disease research?
Microglial cells are crucial for understanding Alzheimer’s disease as they act as the brain’s immune system. They help remove damaged cells and prune synapses, which is essential for healthy brain function. Research by Beth Stevens highlights how improper synaptic pruning by microglia can lead to the progression of Alzheimer’s and other neurodegenerative diseases.
What is the role of synaptic pruning in neurodegenerative diseases like Alzheimer’s?
Synaptic pruning is a process where microglial cells eliminate unnecessary synapses in the brain. In the context of Alzheimer’s disease, researchers like Beth Stevens have discovered that improper pruning may contribute to neurodegeneration. This emphasizes the importance of understanding synaptic mechanisms in Alzheimer’s disease research.
What advances in Alzheimer’s disease treatments have stemmed from microglial research?
Research into microglial cells, particularly by scientists like Beth Stevens, has paved the way for new biomarkers and potential medications for Alzheimer’s disease. By understanding the role of the brain’s immune system in synaptic pruning and damage control, researchers are developing innovative approaches to treat neurodegenerative diseases.
How does Beth Stevens’ research impact our understanding of the brain’s immune system in Alzheimer’s disease?
Beth Stevens’ research has significantly advanced our understanding of how the brain’s immune system, particularly microglial cells, functions in the context of Alzheimer’s disease. Her findings suggest that these cells, when functioning correctly, protect against neurodegeneration, but can exacerbate the disease when their pruning mechanisms go awry.
What are potential biomarkers for Alzheimer’s disease identified through microglial research?
Through the exploration of microglial cells, researchers have identified potential biomarkers linked to Alzheimer’s disease. These biomarkers may help in the early detection of neurodegenerative diseases, highlighting the importance of ongoing research in microglial function and Alzheimer’s pathology.
What challenges are faced in Alzheimer’s disease research related to microglial cells?
Challenges in Alzheimer’s disease research concerning microglial cells include understanding the dual roles these cells play in neuroprotection and neurodegeneration. As highlighted by Beth Stevens, their improper functioning can lead to exacerbated disease conditions, making it crucial to delineate these mechanisms to inform treatment strategies.
What foundational research supports the study of Alzheimer’s disease and microglial function?
Foundational research has been pivotal in advancing the study of Alzheimer’s disease, particularly research that focuses on microglial function. Supported by NIH grants, many breakthroughs have emerged from basic science that explores how these immune cells interact with neuronal processes, leading to greater insights into neurodegenerative diseases.
How can the understanding of microglia influence future Alzheimer’s disease therapies?
Understanding the role of microglia in the context of Alzheimer’s disease can radically influence future therapies. Insights from studies, such as those conducted by Beth Stevens, may lead to targeted treatments that modulate microglial activity to promote synaptic health and reduce neurodegeneration.
| Key Aspect | Details |
|---|---|
| Research Focus | Analysis of microglial cells and their role in Alzheimer’s disease and other disorders. |
| Importance of Microglia | Microglia act as the brain’s immune system, removing damaged cells and pruning synapses. |
| Research Impact | Findings help develop biomarkers and medications for treating neurodegenerative diseases. |
| Funding and Support | Stevens’ research is supported significantly by NIH and federal grants. |
| Long-term Vision | Research may lead to new understandings and treatments for Alzheimer’s disease. |
| Broader Implications | Basic research on microglia can lead to discoveries applicable to human health. |
Summary
Alzheimer’s disease research has revealed critical insights into the role of microglial cells in brain health. By understanding how these cells contribute to synaptic pruning and brain immunity, researchers like Beth Stevens are pioneering new ways to identify and treat neurodegenerative diseases. The ongoing support from institutions like the NIH underscores the value of curiosity-driven science, which often leads to significant advancements in medical knowledge and patient care. As research progresses, we look toward a future where improved treatments can enhance the quality of life for the millions affected by Alzheimer’s disease.

Global Health: Atul Gawande Highlights USAID’s Challenges
Global health is a critical area of concern that transcends national borders, impacting millions around the world. Renowned surgeon Atul Gawande has highlighted the dire consequences of recent cuts to the U.S. Agency for International Development (USAID), emphasizing how these changes threaten public health infrastructure and our position as a global health leader. With research funding cuts leading to diminished capacity for innovation and response to health crises, the implications are staggering. As Gawande and other experts warn, these funding losses are not just administrative; they have real repercussions for communities worldwide who rely on crucial health programs. Engaging in global health initiatives is more vital than ever, as individuals and nations rally to address these significant challenges and preserve the gains made in public health over the past decades.
The realm of international health encompasses a variety of factors that affect the well-being of populations across the globe. It is essential to recognize the impact of governmental decisions, such as the slashing of funds to prominent agencies like USAID, which orchestrate much of our collective public health efforts. As highlighted by health advocates like Atul Gawande, such diminished support affects not just organizational staffing but also the fundamental infrastructure that ensures medical resources reach those in dire need. In addition to the immediate consequences of funding cuts, the broader implications for innovative research and public health strategies become increasingly apparent. The call for renewed commitment to equitable health solutions and robust leadership in this field is more pressing than ever, ensuring that all communities have access to essential health services.
The Impact of USAID Cuts on Global Health Leadership
The recent cuts to the U.S. Agency for International Development (USAID) have caused significant disruption in global health leadership efforts worldwide. Atul Gawande, an influential figure within USAID, highlighted how the agency’s dismantling has led to a detrimental vacuum in support systems that effectively address health crises. With over 85% of USAID programs terminated, the capacity to respond to infectious diseases and maternal health challenges has been severely impaired, raising alarms about the future of global health initiatives. This void undermines the strategic partnerships that USAID established across various countries, essential for monitoring and controlling disease outbreaks.
As Gawande pointed out, this disarray has not only affected international health standards but also hampered America’s role as a respected leader in global health. The capacity to mobilize resources swiftly and offer technical assistance has diminished, threatening the life-saving progress made in countries where USAID had operated. If unchecked, these cuts could reverse decades of advancements in public health, particularly in managing public health infrastructure vital for combating epidemics like HIV and malaria.
Reviving Public Health Infrastructure Post-USAID
The complete overhaul of USAID’s operational framework poses a dire challenge to public health infrastructure, especially in countries that have historically depended on American support. Gawande’s reflection on his time with the agency emphasizes the importance of sustained funding and resources necessary to bolster healthcare systems. Without this foundation, the risk of regress in health outcomes is heightened, particularly concerning maternal and child mortality rates, as well as responses to infectious disease outbreaks that require timely intervention.
Steps must be taken to revive the public health infrastructure that has been critically endangered by recent funding cuts. Community-based health initiatives and collaborations with local organizations are essential to rebuild trust and efficiency in healthcare delivery. Additionally, emphasizing research funding for innovative solutions can pave the way for effective strategies to address persistent health issues impacting vulnerable populations globally, a concern that Gawande urges the public and policymakers to prioritize.
Research Funding Cuts and Their Consequences
The freeze in research funding due to the termination of key federal programs like those from Harvard has led to a chilling effect on scientific advancements in public health. Gawande emphasized that institutions such as the National Institutes of Health play a crucial role in driving research that tackles pressing health issues. Cuts to this funding threaten to hinder groundbreaking studies and innovative medical practices that have improved health outcomes, particularly in marginalized communities often reliant on these advancements for their healthcare.
The implications of reduced research funding extend beyond immediate clinical applications. As Gawande mentioned, the ongoing projects at institutions like Ariadne Labs are at risk. Such research not only tests new surgical techniques and treatments but also informs policy changes that could lead to broader systemic improvements in healthcare delivery. The loss of funding jeopardizes the momentum necessary for making impactful strides in public health initiatives, emphasizing the need for concerted advocacy towards reinstating research support.
Hope for Global Health Amidst Challenges
Despite the daunting challenges faced by global health following the dismantling of USAID, Atul Gawande remains optimistic about the potential for recovery and leadership in the field. He highlighted the resilience of healthcare professionals and advocates who are determined to continue improving health outcomes against significant odds. The commitment to addressing health inequalities and ensuring access to care is more vital now than ever, as new leaders emerge to fill the gaps left by the reductions in U.S. support.
Moreover, Gawande’s emphasis on the proliferation of local leadership reflects a growing trend where countries and organizations are stepping up to claim responsibility for their health agendas. This dynamic shift could lead to innovative solutions tailored to the unique needs of communities globally. The era for America to lead may be waning, but it opens the door for collective global efforts committed to tackling the myriad of health challenges facing the planet.
The Role of Science and Medicine in Recovery
Atul Gawande’s discussions about the essential role of science and medicine underline the importance of maintaining a steadfast commitment to these disciplines in the face of adversity. He encouraged students and faculty to stay engaged and active in their pursuits, regardless of the broader challenges facing the healthcare system. By nurturing a culture of inquiry and innovation, the field can adapt to new realities and reinforce the backbone of global health infrastructure.
In challenging times, the dedication to science becomes crucial in effectively mobilizing efforts to combat health crises. Gawande’s insights highlight how continued investment in education and training within the medical field will equip future leaders with the skills necessary to address emerging health threats. Engaging in research and being vocal proponents for health equity are also vital for meaningful recovery in global health.
The Interdependence of Health and Policy
Gawande’s reflections remind us that health policies must align closely with public health needs to produce tangible outcomes. His experiences illustrate how critical it is to foster collaborations between governmental bodies, healthcare professionals, and academic institutions to ensure that health initiatives are well-funded and effectively implemented. This interconnectedness is essential for achieving long-lasting improvements in health service delivery.
The disruptions caused by funding cuts have also revealed the need for increased advocacy for policies that support sustainable health frameworks. Gawande advocates for an informed and engaged public that holds policymakers accountable for decisions impacting health outcomes. As the landscape of global health continues to evolve, ongoing dialogue and advocacy will play pivotal roles in shaping a more equitable health future.
The Importance of Global Health Partnerships
Partnerships across borders have historically led to some of the most successful public health interventions. As Gawande noted, effective health action often relies on collaborative efforts that leverage diverse resources and expertise. Strengthening these partnerships can serve as a catalyst for innovative solutions to pressing health challenges, from epidemic response to maternal health improvement.
Global health initiatives must not only focus on immediate outcomes but also build long-term relationships that empower local health systems. By harnessing the strengths of various stakeholders—governments, nonprofits, academic institutions, and local communities—health strategies can be tailored to meet specific needs, ultimately leading to enhanced health resilience in the face of global challenges.
Emerging Trends in Global Health Leadership
As the landscape of global health shifts due to reduced U.S. influence, new trends in leadership are emerging. Gawande points to the rise of localized leadership scenarios where countries take charge of their health dilemmas. This represents a critical transition towards self-sufficiency in health policies and implementations, fostering a sense of accountability and ownership among nations.
Moreover, the ability of emerging leaders to navigate and adapt to changing circumstances is essential in maintaining momentum in global health efforts. These leaders often prioritize innovative strategies and collaborations that can better address the unique needs of their populations, ensuring that global health ambitions remain attainable even amidst challenging times.
Rebuilding Trust in Public Health Systems
Trust is a cornerstone of effective public health systems, and the cuts to USAID showcased vulnerabilities that can disrupt community confidence in health initiatives. Gawande emphasizes the importance of transparent communication and demonstrated efficacy in rebuilding this trust. Public perception can significantly influence the success of health programs, making it essential for leaders to engage communities in health decision-making.
To restore trust, partnerships that prioritize community involvement must be established. Efforts to listen to the needs and concerns of local populations can drive appropriate interventions, ultimately enhancing the effectiveness of public health efforts. Emphasis on community-led solutions can bridge gaps that funding cuts have introduced, fostering resilience and adaptability in health systems globally.
Frequently Asked Questions
What impact did Atul Gawande highlight regarding the cuts to USAID on global health?
Atul Gawande emphasized that the cuts to USAID inflected ‘devastating’ harm on global health, drastically reducing the capacity to monitor and respond to disease outbreaks and undermining health care support for millions, especially in maternal and child health. The dismantling of USAID’s programs has severely affected public health infrastructure in partnering countries, increasing the risk of outbreaks and health disparities.
How do USAID funding cuts affect public health infrastructure globally?
The cuts to USAID directly weaken public health infrastructure in countries reliant on U.S. support for disease control and health programs. These reductions have led to cancellations of vital projects aimed at reducing diseases such as HIV, tuberculosis, and malaria, thus jeopardizing the achievements made in improving global health outcomes.
How can cuts to research funding impact global health initiatives?
Cuts to research funding can stall innovation and effectiveness in global health initiatives. Reduced funding for organizations like the National Institutes of Health and CDC hampers crucial research necessary for developing and improving interventions for pressing global health issues, such as maternal mortality and infectious diseases.
Why is USAID’s role critical in addressing global health challenges?
USAID plays a crucial role in addressing global health challenges by providing technical assistance and resources that enhance public health responses, improve vaccination coverage, and ensure timely interventions during health crises. Its ability to maintain partnerships and programs globally is vital for sustaining progress in health outcomes, particularly in developing regions.
What alternatives exist if the U.S. withdraws from its role in global health leadership?
If the U.S. withdraws from its role in global health leadership, other countries and regional organizations are likely to step up, potentially shifting the dynamics of global health partnerships. It will require a collaborative effort by global leaders to fill the void and ensure essential health services continue, especially in underserved populations.
What innovative services was USAID poised to implement before the funding cuts?
Before the funding cuts, USAID was set to implement an innovative treatment package aimed at reducing severe hemorrhaging post-childbirth, which is the leading cause of maternal deaths worldwide. This initiative reflected USAID’s commitment to improving maternal health outcomes through effective, evidence-based interventions.
How has the funding freeze affected ongoing global health research efforts?
The funding freeze has negatively impacted ongoing global health research capabilities, notably affecting centers like Ariadne Labs. This jeopardizes critical research and projects that seek to enhance maternal health, surgical safety, and primary care, thus delaying advancements that could significantly benefit global health outcomes.
| Key Points | Details |
|---|---|
| Atul Gawande’s Experience | Gawande, surgeon and author, served as head of USAID’s Bureau for Global Health and criticized the dismantling of USAID. |
| Impact of USAID Cuts | Termination of staff and programs resulted in severe health deficits globally, affecting millions and diminishing US global health leadership. |
| Achievements of USAID | USAID implemented programs that significantly improved health outcomes across various disease sectors like maternal health and infectious diseases. |
| Gawande’s Perspective on Future | Despite setbacks, Gawande remains hopeful about the future of global health, stressing the importance of ongoing commitment to health work. |
| Call to Action | Gawande urges future experts to remain engaged in global health despite uncertainties. |
Summary
Global health is becoming increasingly critical as multiple crises threaten health infrastructures worldwide. Atul Gawande’s insights highlight the significant setbacks caused by funding cuts and staff terminations at USAID, warning that repairing the damage will require dedicated effort and commitment to science and public health. As we face these challenges, it is essential to remember that the work in global health is more crucial than ever, and the involvement of skilled individuals in this field will be vital for overcoming current and future health crises.

Pediatric Cancer Recurrence Prediction with AI Insights
Pediatric cancer recurrence prediction is a groundbreaking area of research that harnesses the power of artificial intelligence (AI) to enhance patient care. Recent studies have demonstrated that AI tools can analyze a patient’s brain scans over time, significantly improving the prediction of relapse risks in children suffering from brain tumors such as gliomas. Unlike traditional methods that often fall short, this innovative approach utilizes machine learning to monitor brain tumor progression more accurately. By employing techniques like temporal learning in healthcare, researchers can synthesize findings from multiple imaging sessions, ultimately providing clearer insights into the likelihood of a child’s cancer returning. As the landscape of pediatric oncology evolves, the potential of AI in pediatric cancer opens doors to better management strategies and improved outcomes for young patients.
The field of predicting cancer recurrence in children has seen a transformative shift thanks to advances in technology. With tools that leverage AI capabilities, healthcare providers are now able to gauge glioma relapse risk more effectively than ever before. This method relies on understanding how tumor characteristics change over time, owing to devices that monitor brain tumors and automatically analyze multiple scans. Terms like machine learning cancer prediction and brain tumor monitoring are becoming synonymous with the effort to enhance treatment precision for pediatric patients. Through innovative methodologies such as these, the future of oncology holds promise in providing children with more tailored treatment pathways.
The Evolution of AI in Pediatric Cancer Care
The integration of artificial intelligence (AI) in pediatric cancer care marks a significant turning point in how medical professionals manage and predict the course of illnesses. Traditionally, treatment plans relied heavily on clinician experience and outdated predictive models that often lacked precision. Now, with advancements in technology, machine learning algorithms can analyze extensive datasets, including past patient records and imaging scans, to identify patterns that may indicate disease progression or recurrence. This shift not only enhances decision-making for healthcare providers but also offers families more accurate prognoses.
The application of AI in pediatric cancer care extends beyond simple data analysis; it paves the way for innovative predictive tools that incorporate temporal learning, enabling more dynamic monitoring of a child’s health. By utilizing algorithms trained on longitudinal data—from multiple imaging sessions over time—clinicians can develop a clearer understanding of patient trajectories. Such techniques create a more nuanced picture of the patient’s response to treatment, ultimately elevating the standard of care.
Understanding Glioma Relapse Risk with AI Technology
One of the most challenging aspects of treating pediatric gliomas is predicting the risk of relapse. These brain tumors, though often treatable, can return unexpectedly, leading to secondary health complications and psychological distress for the patients and their families. The emerging AI tools utilize extensive imaging datasets and temporal learning to enhance the reliability of these predictions significantly. By aggregating data from hundreds of pediatric patients, researchers are learning to identify those most at risk for recurrence with accuracies that challenge traditional methods.
This progress in understanding glioma relapse risk not only serves to bolster clinical insights but also radically transforms patient follow-up processes. As AI continues to evolve, the hope is that it will facilitate personalized treatment plans, allowing for tailored interventions that can either decrease the frequency of MRI scans for lower-risk patients or prompt preemptive therapy for those flagged as high-risk. This focused approach reduces unnecessary stress and resource expenditures, optimizing the management of pediatric cancer.
Harnessing Temporal Learning for Better Predictions
Temporal learning is a groundbreaking technique that has significant implications for the future of healthcare, particularly in oncology. By analyzing a series of brain scans taken over time rather than relying on single scans, AI models can uncover critical changes in tumor characteristics that may indicate an increased risk of recurrence. This innovative approach allows healthcare providers to benefit from a comprehensive view of the disease progression, facilitating more informed medical decisions.
By implementing temporal learning, researchers have seen a marked improvement in predicting glioma recurrence rates among pediatric patients, with accuracy levels between 75-89%. This finding highlights the potential for AI-driven decisions to better inform treatment pathways. The excitement surrounding these advancements underscores the promise of machine learning cancer prediction not just for gliomas but for various forms of pediatric cancer, creating standardized methods of monitoring that could herald a new era in cancer care.
Challenges and Future Directions in AI Healthcare Applications
While the potential for AI in predicting pediatric cancer recurrence, such as in gliomas, is promising, several challenges remain. The need for further validation in diverse clinical environments is paramount to ensure the effectiveness and reliability of these models. Additionally, achieving a broad consensus within the medical community regarding the interpretation and clinical application of AI-derived predictions will be crucial for widespread adoption. Ethical considerations also arise, particularly concerning data privacy and the implications of algorithmic decision-making in sensitive health contexts.
Looking ahead, the journey of integrating AI into pediatric cancer monitoring and treatment is just starting. Ongoing collaborative efforts among research institutions, hospitals, and technology firms are essential to refine these AI tools further. By addressing existing concerns and continuously improving predictive algorithms, the healthcare industry stands to transform patient outcomes for children battling cancer, marking a transition from reactive treatment models to proactive risk management.
Machine Learning Techniques in Cancer Research
Machine learning techniques have revolutionized cancer research by offering computational power that enhances the analysis of complex datasets. In pediatric oncology, these algorithms sift through vast amounts of medical data, including previous treatment outcomes and demographic factors, to identify subtle trends and risk factors that may not be evident through traditional analysis. By employing these advanced techniques, researchers can develop predictive models that not only forecast treatment success rates but also uncover personalized patient needs.
As machine learning continues to evolve, its potential applications expand across various facets of cancer care—from diagnosis to treatment evaluation. AI in pediatric cancer, particularly with a focus on recurring conditions, allows for ongoing learning and adaptation of treatment protocols based on real-time data analytics. This method enhances not only the accuracy of predictions regarding risks associated with glioma and other tumors but also optimizes overall healthcare delivery.
The Role of MRI in Longitudinal Cancer Monitoring
Magnetic Resonance Imaging (MRI) plays a pivotal role in the landscape of pediatric cancer monitoring, offering detailed insights into tumor behavior and treatment efficacy over time. Its ability to provide high-resolution images of the brain allows clinicians to observe changes that may signify recurrence of gliomas. The challenge in traditional imaging protocols lies in the frequency and duration of follow-up scans, which can burden young patients and their families both emotionally and financially.
The infusion of AI technology into MRI analysis significantly enhances the interpretive capabilities of these scans, where algorithms can pinpoint minute shifts in tumor structure that might be missed by the human eye. Consequently, physicians can make more informed decisions regarding patient care, ensuring that children who are at high risk for recurrence receive appropriate follow-ups, while also alleviating the imaging burden for lower-risk patients. This reprioritization of healthcare resources optimizes patient wellbeing, allowing for a more focused, evidence-based approach.
Clinical Trials: Testing AI’s Real-World Impact
The transition from research findings to clinical application is a critical step in realizing the benefits of AI in healthcare, especially in predicting pediatric cancer recurrence. Ongoing clinical trials are essential to validate the efficacy and safety of AI-driven predictive models, particularly those utilizing temporal learning techniques. Such trials will explore whether the application of these innovative tools can truly enhance patient outcomes in real-world settings.
Participating in clinical trials also provides valuable data that can refine predictive models even further. Assessing AI predictions against actual patient outcomes allows researchers to adjust algorithms accordingly, leading to continual enhancements in accuracy and reliability over time. Ultimately, the successful integration of AI tools into pediatric oncology will rely on rigorous testing and validation, translating technological advancements into tangible benefits for children facing cancer.
Integrating AI in Pediatric Oncology: A Team Effort
The integration of AI in pediatric oncology is a multifaceted endeavor requiring collaboration among researchers, clinicians, data scientists, and healthcare administrators. As the field evolves, interdisciplinary teams will become crucial to ensure that AI tools are not only efficient but also seamlessly fit within the existing healthcare structures. Moreover, engaging families and patients throughout the development process empowers them with insight into how AI-driven predictions will be applied to their care.
Furthermore, fostering partnerships with technology firms and academic institutions is vital for advancing machine learning initiatives in pediatric cancer research. This teamwork can usher in innovative solutions, addressing pressing challenges such as data privacy, algorithm accuracy, and patient accessibility. By harmonizing the strengths of various stakeholders, the healthcare community can cultivate a future where AI resources effectively support pediatric oncology, enhancing outcomes and quality of care for vulnerable populations.
The Future of Brain Tumor Monitoring with AI
As research progresses, the future of brain tumor monitoring stands to be transformed by AI technologies. The potential for more effective surveillance approaches that leverage machine learning insights provides a glimmer of hope for pediatric patients diagnosed with gliomas. By utilizing AI-driven predictive models, physicians can offer patients tailored follow-up care that is better aligned with their individual risk profiles, minimizing unnecessary imaging while still being vigilant against potential relapses.
Ultimately, the future of pediatric cancer monitoring lies in harnessing the capabilities of AI to forge a more precise and compassionate care experience for young patients. Through ongoing innovation and testing of these technologies, there is a sincere hope that AI will positively impact not only the lives of children facing cancer but also the families supporting them through this journey.
Frequently Asked Questions
What is pediatric cancer recurrence prediction and how is it improving with AI?
Pediatric cancer recurrence prediction refers to methods used to anticipate the likelihood of cancer returning in children after treatment. Recent advancements have shown that AI tools significantly enhance this prediction ability by analyzing multiple brain scans over time. A study from Mass General Brigham demonstrated that AI, particularly through techniques like temporal learning, provides more accurate relapse risk assessments for pediatric gliomas compared to traditional methods.
How does AI analyze pediatric cancer relapse risk better than traditional methods?
AI enhances pediatric cancer relapse risk analysis by utilizing a method called temporal learning, which allows it to evaluate multiple brain scans taken over time rather than just individual images. This approach enables the model to detect subtle changes in a patient’s condition, thereby improving its predictions regarding glioma relapse risk with an accuracy between 75%-89%.
What role does temporal learning play in pediatric cancer recurrence prediction?
Temporal learning plays a crucial role in pediatric cancer recurrence prediction by training AI models to synthesize information from a series of MR scans. Instead of relying on single scans, this method allows the AI to recognize patterns and trends over time, leading to more reliable forecasts of whether a child is at high risk for glioma relapse.
Can machine learning improve outcomes for pediatric glioma patients?
Yes, machine learning has the potential to significantly improve outcomes for pediatric glioma patients by providing more precise predictions on cancer recurrence. By utilizing advanced algorithms and analyzing longitudinal imaging through temporal learning, healthcare providers can better stratify patients based on their relapse risk and optimize their treatment plans accordingly.
What benefits does AI in pediatric cancer offer families?
AI in pediatric cancer, especially in recurrence prediction, offers families several benefits, including reduced frequency of MR imaging for low-risk patients, minimizing stress and burden during follow-ups. Additionally, early identification of high-risk cases allows for timely intervention with targeted therapies, aiming to improve long-term health outcomes for children fighting gliomas.
How accurate is the AI prediction for glioma relapse risk post-treatment?
The AI prediction for glioma relapse risk post-treatment has shown an impressive accuracy range of 75%-89% when utilizing temporal learning techniques. This accuracy surpasses traditional prediction methods based on single images, which only achieved around 50% accuracy, making AI a promising advancement in the monitoring of pediatric cancer patients.
What future developments are expected in pediatric cancer monitoring with AI?
Future developments in pediatric cancer monitoring with AI are anticipated to include the validation of temporal learning models across various clinical settings and the potential launch of clinical trials. These advancements could lead to personalized treatment plans, such as tailored imaging schedules for low-risk patients and enhanced therapeutic interventions for those identified as high-risk for glioma recurrence.
What is the significance of multi-scan analysis in pediatric cancer predictions?
Multi-scan analysis is significant in pediatric cancer predictions as it allows AI to evaluate a patient’s health over time, capturing changes that a single scan might miss. This comprehensive approach leads to more accurate assessments of glioma relapse risk, facilitating better-informed clinical decisions and improving patient outcomes.
| Key Point | Details |
|---|---|
| AI Predicts Recurrence | An AI tool predicts relapse risk in pediatric glioma patients more accurately than traditional methods. |
| Research Collaboration | Study conducted by Mass General Brigham, Boston Children’s Hospital, and Dana-Farber/Boston Children’s Cancer and Blood Disorders Center. |
| Temporal Learning Technique | AI uses temporal learning to analyze multiple brain scans over time, improving prediction of cancer recurrence. |
| Improved Accuracy | The model achieved a prediction accuracy of 75%-89%, significantly better than the typical 50% from single scans. |
| Future Directions | Further validation needed; clinical trials aimed at improving care for pediatric glioma patients are planned. |
Summary
Pediatric cancer recurrence prediction is becoming increasingly sophisticated with the development of AI tools that enhance prediction accuracy significantly. Traditional methods have struggled to provide timely alerts for relapses in pediatric patients with gliomas. However, recent research indicates that AI, through innovative techniques like temporal learning, can analyze multiple brain scans over time to foresee potential relapses more effectively. This progression not only paves the way for earlier intervention and better management strategies but also reduces the burden of frequent imaging on families. As further trials unfold, the potential for AI to revolutionize pediatric cancer care looks promising.